
by admin | Jun 30, 2023 | Health Awareness
What is Foot Drop?
Foot drop refers to a neuromuscular condition that affects the muscles and nerves responsible for controlling the movement of the foot. It causes difficulty or inability to lift the front part of the foot, resulting in dragging or scuffing of the foot while walking. This condition can affect one or both feet and can significantly impact a person’s mobility and quality of life.
Foot drop is typically caused by weakness or paralysis of the muscles responsible for lifting the foot, as well as impairment of the nerves that control these muscles. The muscles involved in foot dorsiflexion (lifting the foot) are primarily located in the anterior compartment of the lower leg and are innervated by the peroneal nerve. When the peroneal nerve is damaged or its function is disrupted, the foot may hang downward and the person may have difficulty lifting the foot during walking.
Causes of Foot Drop
There are several potential causes of foot drop, including:
Nerve Compression: Conditions such as herniated discs, spinal stenosis, or peripheral nerve injuries can compress the nerves that control foot movement, leading to foot drop.
Muscular Disorders: Certain muscular disorders, such as muscular dystrophy or Charcot-Marie-Tooth disease, can weaken the muscles responsible for foot movement, causing foot drop.
Neurological Conditions: Neurological disorders like stroke, multiple sclerosis, or cerebral palsy can damage the nerves that control foot muscles, resulting in foot drop.
Trauma or Injury: Injuries to the spinal cord, lower back, or leg can disrupt nerve signals and lead to foot drop.
Side Effects of Medications: Certain medications, such as those used in chemotherapy, can cause peripheral neuropathy, which may result in foot drop as a side effect.
Symptoms and Diagnosis
The primary symptom of foot drop is difficulty lifting the front part of the foot, causing it to drag or slap the ground while walking. Other associated symptoms may include weakness in the affected leg, numbness or tingling in the foot or lower leg, loss of balance while walking, and pain or discomfort in the foot or leg.
A medical professional can diagnose foot drop by conducting a thorough physical examination, reviewing the patient’s medical history, and ordering additional tests such as nerve conduction studies or imaging scans.
What are Treatment Options?
The treatment for foot drop depends on the underlying cause and severity of the condition. Some common treatment options include:
Physical Therapy: Physical therapy exercises can help improve muscle strength and flexibility, as well as enhance gait and walking abilities.
Assistive Devices: The use of orthotic devices, such as braces or splints, can provide support to the foot and help maintain a more natural walking pattern.
Nerve Stimulation: Electrical nerve stimulation techniques, such as functional electrical stimulation (FES), can help activate the muscles and improve foot movement.
Surgery: In severe cases or when other treatment options are ineffective, surgical intervention may be considered. Surgical procedures aim to address the underlying cause of foot drop, such as nerve decompression or tendon transfer.
Medications: Medications may be prescribed to manage pain or treat the underlying condition causing foot drop, such as anti-inflammatory drugs or muscle relaxants.
Regenerative Medicine: Mesenchymal stem cells (MSCs) have the ability to differentiate into nerve cells and release growth factors that promote nerve regeneration, potentially helping to repair damaged nerves associated with foot drop. MSCs possess anti-inflammatory properties and can help reduce inflammation in the affected area, which may contribute to the recovery and healing process. MSCs can secrete various bioactive molecules that support tissue repair, angiogenesis (formation of new blood vessels), and tissue regeneration, which could aid in the restoration of normal foot function.
Talk to a qualified healthcare professional who specializes in regenerative medicine or stem cell therapies. They can help you in making an informed decision based on your individual circumstances.

by admin | Jun 28, 2023 | Mesenchymal Stem Cells, Stem Cell Research, Stem Cell Therapy
Cigarette smoking continues to be the leading contributor to preventable disease and death in the United States, including cancer, heart disease, stroke, lung diseases, diabetes, and chronic obstructive pulmonary disease (COPD). Smoking cigarettes also increases the risk of tuberculosis, certain eye diseases, and problems of the immune system, including rheumatoid arthritis.
An abundance of clinical research has clearly shown the detrimental effects cigarette smoke has on nearly every area of the body. However, while assumed to be equally dangerous in its effect on stem cells, there is surprisingly little research exploring the negative implications of cigarette smoking on stem cells.
In this review, Nguyen et al. share findings of recent studies on the effects of cigarette smoking and nicotine on mesenchymal stem cells (MSCs), with a specific focus on dental stem cells.
With their ability to self-renew, develop into specialized cell types, and migrate to potential sites of injury, stem cells have demonstrated the potential to build every tissue in the body and have also demonstrated great potential for tissue regeneration and associated therapeutic uses.
As the potential benefits and weaknesses of stem cells continue to be discovered, researchers have found that cigarette smoking negatively impacts the abilities of stem cells while also limiting stem cell viability for transplantation and regeneration.
While there has been a recent decline in the percentage of U.S. adults who smoke, over 34 million U.S. adults continue to be regular cigarette smokers. Interestingly, research has demonstrated the concentration of nicotine to be significantly higher in saliva than in blood plasma following nicotine administration via cigarette, e-cigarette, and nicotine patch – in some cases measuring up to eight times higher concentrations. Considering this research and considering the established detrimental effects of e-cigarette vapor – and presumably nicotine – on teeth and dental implants, the authors of this review hypothesized that there would be a similar effect when dental stem cells are exposed to cigarette smoke.
Reviewing the effect that cigarette smoke has on MSCs, the authors found that exposing MSCs to cigarette smoke extract (CSE) and nicotine impaired cell migration, increased early and late osteogenic differentiation markers, decreased cell proliferation, and significantly inhibited the ability of MSCs to differentiate to other types of cells.
Nguyen et al. reviewed research that determined cigarette smoke produced a negative impact on the proliferation and differentiation of dental pulp stem cells (DPSCs). Specifically, this research demonstrated a significantly higher depression of alkaline phosphatase (ALP) and osteocalcin (OC) genes in smokers when compared to nonsmokers. Additional studies found that smokers demonstrated reduced calcium deposition levels and production of ALP when compared to nonsmokers.
Cigarette smoke and nicotine were also found to negatively affect the migration capability of dental stem cells, slowing the migration rate by up to 12% in smokers while also producing a smaller reduction of scratch wound areas when compared to nonsmokers.
While there are not many studies directly comparing the effects of cigarette smoke and nicotine on MSCs and dental stem cells, the authors conclude that dental stem cells exhibit similar characteristics to bone marrow MSCs and that both of these types of stem cells demonstrate similar negative responses upon their exposure to nicotine.
While the authors call for further research to better understand the specific effects of cigarette smoke on dental stem cells, the authors conclude that the findings demonstrating similar responses to cigarette smoke and nicotine between dental stem cells and MSCs can be used to inform future dental stem cell studies. These findings will help dentists better identify which patients might be at an increased risk of poor healing in the oral cavity and if smoking cessation should be considered before undergoing any invasive or traumatic dental procedure, such as tooth extraction.
Source: Comparison of the effect of cigarette smoke on mesenchymal stem ….” https://journals.physiology.org/doi/10.1152/ajpcell.00217.2020.

by Stemedix | Jun 26, 2023 | Degenerative Disc Disease
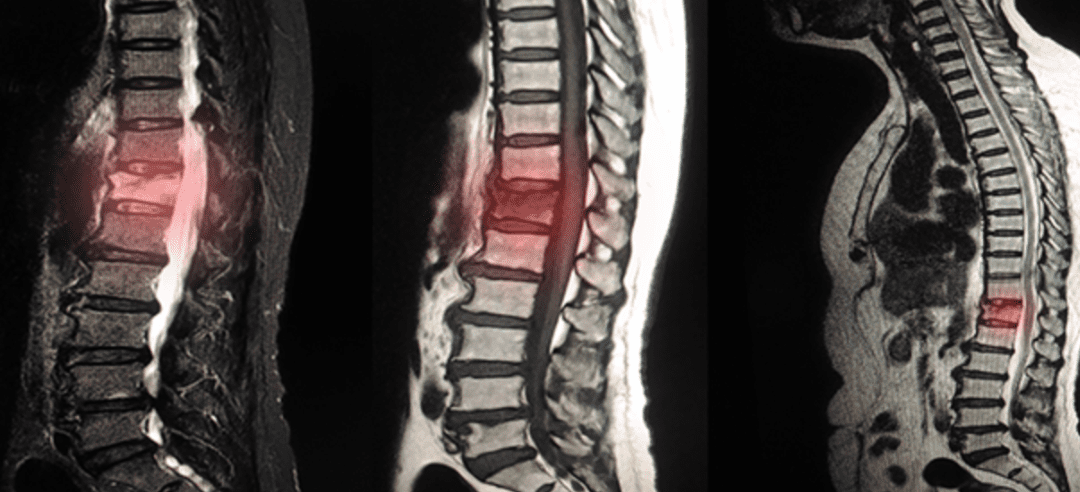
What is Degenerative Disc Disease?
Degenerative disc disease (DDD) is a condition that refers to the gradual deterioration of the discs between the vertebrae of the spine. The discs are rubbery cushions that act as shock absorbers and provide flexibility to the spine. With age and wear and tear, these discs can degenerate, leading to various symptoms and changes in the spine. In this article, we will discuss the things to avoid with degenerative disc disease.
The primary cause of degenerative disc disease is the natural aging process. As we age, the discs lose their water content and become less flexible, resulting in decreased disc height and integrity. This degeneration can also be influenced by factors such as genetics, lifestyle, and repetitive stress on the spine.
The common symptoms of degenerative disc disease include back pain, neck pain, and radiating pain or numbness that can extend into the arms or legs. The pain may worsen with activities like bending, twisting, or sitting for prolonged periods. In some cases, the degenerated disc may impinge on nearby nerves, causing additional symptoms such as weakness or tingling sensations in the affected areas.
While degenerative disc disease is a natural part of the aging process, not everyone with disc degeneration experiences symptoms. The severity of symptoms can vary widely among individuals. Diagnosis of degenerative disc disease typically involves a combination of medical history, physical examination, and imaging tests such as X-rays, MRI scans, or CT scans to assess the condition of the discs and rule out other possible causes of pain.
Who Do You See if You Think You Have Degenerative Disc Disease?
If you suspect that you have degenerative disc disease (DDD), it is advisable to consult with a healthcare professional who specializes in spine conditions. Here are some healthcare providers you can consider seeing for an evaluation and diagnosis:
Primary Care Physician (PCP): Start by scheduling an appointment with your primary care physician. They can assess your symptoms, perform a physical examination, and provide initial guidance. They may also refer you to a specialist for further evaluation if needed.
Orthopedic Surgeon: An orthopedic surgeon specializes in the musculoskeletal system, including conditions related to the spine. They can evaluate your symptoms, order appropriate diagnostic tests, and discuss treatment options ranging from conservative approaches to surgical interventions.
Neurologist: Neurologists are medical doctors who specialize in conditions related to the nervous system, including spine-related issues. They can evaluate your symptoms, perform neurological examinations, and order imaging tests to help diagnose degenerative disc disease. They can also provide recommendations for treatment and management.
Spine Specialist: A spine specialist, such as a physiatrist or a spine surgeon, focuses specifically on spine-related conditions. They have expertise in evaluating and treating degenerative disc disease. They can provide a comprehensive evaluation, recommend appropriate diagnostic tests, and develop a tailored treatment plan based on your specific needs.
Physical Therapist: Physical therapists can play a crucial role in managing degenerative disc disease. They can assess your condition, develop an exercise program to strengthen the muscles supporting your spine, and provide guidance on proper body mechanics and posture.
It is important to note that the availability of these specialists may vary depending on your location and healthcare system. In some cases, your primary care physician may provide sufficient guidance and refer you to the appropriate specialist if necessary. Seeking professional medical advice is crucial for an accurate diagnosis and to develop an effective treatment plan tailored to your individual needs.
Things to Avoid with Degenerative Disc Disease
When living with degenerative disc disease, it is important to be mindful of certain activities and habits that can worsen your symptoms or potentially harm your spine.
Firstly, heavy lifting should be avoided as it places excessive strain on your discs. If lifting is necessary, remember to use proper techniques and ask for assistance when needed.
Prolonged sitting or standing should also be minimized, as both positions can place stress on your discs. Instead, try to alternate between sitting and standing and incorporate short breaks or walks throughout the day.
High-impact activities such as running, jumping, or contact sports should be avoided, as they can further deteriorate your discs. Opt for low-impact exercises like swimming or cycling, which are gentler on your spine. Repetitive activities such as bending, twisting, or lifting should be minimized or balanced with frequent breaks to reduce strain on your discs. A sedentary lifestyle weakens the supporting muscles of your spine, so engage in regular physical activity and exercises that promote spinal health.
Maintaining good posture is crucial; avoid slouching or hunching over, especially during extended periods of sitting or standing. Use ergonomic chairs or supportive cushions to help maintain proper alignment.
Smoking is detrimental to your spinal health, so it is advisable to quit smoking or avoid exposure to secondhand smoke.
Excess body weight adds strain to your spine and accelerates disc degeneration, so maintaining a healthy weight through a balanced diet and regular exercise is essential.
Emotional stress and poor sleep can increase muscle tension and exacerbate pain associated with degenerative disc disease, so prioritize stress management techniques and ensure you get enough restful sleep.
It is always recommended to consult with your healthcare provider for personalized advice and to develop a comprehensive treatment plan tailored to your specific condition.
What are Treatment Options for Degenerative Disc Disease?
Traditional treatment options for degenerative disc disease aim to manage pain, improve function, and prevent further deterioration. Conservative measures include physical therapy, pain medications, hot or cold therapy, and lifestyle modifications such as maintaining a healthy weight and adopting proper body mechanics. In more severe cases, when conservative treatments fail to provide relief, surgical interventions such as spinal fusion or artificial disc replacement may be considered.
It’s important to note that degenerative disc disease is a chronic condition, and while traditional treatment can help manage symptoms, it may not reverse the underlying degeneration.
Regenerative Medicine for Degenerative Disc Disease
Regenerative medicine, also known as stem cell therapy, is an emerging field that explores innovative treatments aimed at stimulating the body’s natural healing and regenerative processes.
Mesenchymal stem cell (MSC) therapy is a regenerative medicine approach that has gained attention for its potential in treating degenerative disc disease (DDD). MSCs are a type of adult stem cell that can differentiate into various cell types, including those found in intervertebral discs. These cells are administered to the targeted disc(s) to promote regeneration and repair.
Clinical studies and preliminary research on MSC therapy for DDD have shown promising results. Some potential benefits observed include decreased pain, improved disc hydration, increased disc height, and enhanced structural integrity. Consult with a qualified healthcare professional who specializes in regenerative medicine or spine conditions to discuss the potential benefits, risks, and availability of MSC therapy for degenerative disc disease. They can evaluate your specific case, and provide personalized recommendations based on your individual needs. To learn more about things to avoid with Degenerative Disc Disease, contact us today at Stemedix!

by Stemedix | Jun 19, 2023 | Mesenchymal Stem Cells, Athletic Injury
A very common question we get is ” How long does Stem Cell Therapy last for Knees? ” for those seeking this alternative treatment for the management of their knee pain. But first, we will discuss what can be behind the knee pain as a cause, who to seek a medical diagnosis, and what options a patient has.
What Can Cause Knee Pain?
Knee pain can have various causes, leading to discomfort and limitations in daily activities. One common cause is injuries, which can occur from sudden trauma or repetitive strain. Sprains, strains, ligament tears (like the anterior cruciate ligament or ACL tears), meniscus tears, fractures, or dislocations can result in knee pain.
Degenerative conditions like osteoarthritis often affect the knee joint. Over time, the protective cartilage on the ends of the bones wears down, causing pain, stiffness, and swelling. Rheumatoid arthritis, an autoimmune disease, can also lead to knee pain due to joint inflammation.
Tendinitis, characterized by inflammation or irritation of the tendons around the knee, is typically caused by overuse or repetitive stress. Bursitis, another inflammatory condition, occurs when the small fluid-filled sacs (bursae) between bones, tendons, and muscles become inflamed.
Patellofemoral pain syndrome refers to pain around or behind the kneecap and is often caused by overuse, muscle imbalances, or improper tracking of the kneecap. IT band syndrome, on the other hand, arises from irritation or inflammation of the IT band along the outer thigh and can cause outer knee pain.
Conditions such as gout, marked by the accumulation of uric acid crystals in the joints, can lead to sudden and severe knee pain, redness, and swelling. Infections, though rare, can also cause knee pain, with symptoms including warmth, redness, and swelling.
Additional factors contributing to knee pain include ligamentous or muscular strains, bone tumors, obesity, poor biomechanics, or referred pain from other parts of the body.
If you are experiencing persistent or worsening knee pain, it is crucial to consult with a healthcare professional for a proper diagnosis so to better help the treatment planning.
Who Do I See if I Have Knee Pain?
If you have knee pain, there are several healthcare professionals you can consult with for evaluation, diagnosis, and treatment. The appropriate healthcare provider may depend on your specific situation and the severity of your knee pain. Here are some specialists who commonly deal with knee-related issues:
Primary care physician (PCP): Your first step is often to see your primary care physician. They can assess your knee pain, perform a physical examination, and provide initial treatment or refer you to a specialist if needed.
Orthopedic specialist: Orthopedic doctors specialize in the musculoskeletal system and commonly treat knee pain and related conditions. They can diagnose the underlying cause of your knee pain, recommend imaging tests, if necessary (such as X-rays or MRI), and provide both nonsurgical and surgical treatment options.
Rheumatologist: If your knee pain is suspected to be related to inflammatory or autoimmune conditions like rheumatoid arthritis, a rheumatologist can provide expertise in diagnosing and managing such conditions.
Sports medicine specialist: These specialists focus on injuries and conditions related to sports and physical activity. If your knee pain is sports-related or if you have an active lifestyle, a sports medicine specialist can help with diagnosis, treatment, and rehabilitation.
Physical therapist: Physical therapists can be involved in the treatment of knee pain, especially for rehabilitation and strengthening exercises. They can provide exercises, stretches, and techniques to improve knee function and reduce pain.
Pain management specialist: If your knee pain is chronic and not easily managed with conventional treatments, a pain management specialist can provide additional options such as medications, injections, or other interventional procedures to alleviate pain.
Are There Alternative Medicine Treatments for Helping with Knee Pain?
Yes, there are alternative medicine treatments that some individuals may consider for helping with knee pain. These alternative approaches focus on holistic and natural methods to address pain and promote overall well-being. While they may not be suitable or effective for everyone, some people find them helpful as complementary or adjunct therapies. Here are a few alternative medicine treatments that are sometimes used for knee pain:
Acupuncture: This ancient Chinese practice involves the insertion of thin needles into specific points on the body. Acupuncture is believed to stimulate energy flow and promote pain relief and healing. Some people report reduced knee pain and improved function with acupuncture.
Herbal remedies: Certain herbs and botanicals are believed to have anti-inflammatory properties and can be used topically or taken orally to alleviate knee pain. Examples include turmeric, ginger, Boswellia, and willow bark. However, it’s essential to consult with a qualified herbalist or healthcare provider before using any herbal remedies, as they can interact with medications and have potential side effects.
Topical creams and ointments: Various topical preparations containing natural ingredients like arnica, menthol, capsaicin, or essential oils are available and can be applied to the knee to provide temporary relief from pain and inflammation.
Mind-body techniques: Practices such as meditation, mindfulness, yoga, and tai chi can help manage knee pain by promoting relaxation, reducing stress, improving flexibility, and enhancing body awareness. These techniques may also improve overall physical and mental well-being.
Physical therapies: Alternative physical therapies like chiropractic care, osteopathy, or naturopathy may incorporate manual techniques, stretching, manipulation, or mobilization to address knee pain. These approaches often aim to enhance joint mobility, improve alignment, and reduce pain.
Regenerative Medicine: Also known as stem cell therapy, this regenerative medicine utilizes mesenchymal stem cells (MSCs) for joint pain by promoting healing, repair, and regeneration of damaged joint tissues.
What is MSC Therapy for Knee Pain?
MSC (mesenchymal stem cell) therapy is a form of regenerative medicine that has gained attention as a potential treatment for knee pain and knee-related conditions. MSCs are multipotent cells that have the ability to differentiate into various cell types, including bone, cartilage, and fat cells. They also possess anti-inflammatory and immunomodulatory properties. Most people ask the question of ” How long does Stem Cell Therapy for knees last? ” With MSC Therapy for Knee Pain in mind.
The goal of MSC therapy is to promote tissue regeneration, reduce inflammation, and potentially slow down the progression of conditions such as osteoarthritis. By injecting MSCs into the knee joint, it is believed that the cells can stimulate the repair of damaged tissues, enhance cartilage regeneration, and modulate the immune response, thereby reducing pain and improving function.If you are considering MSC therapy for knee pain, it is essential to consult with a qualified healthcare professional who specializes in regenerative medicine. They can assess your specific situation, discuss the potential benefits and risks, and provide guidance on whether MSC therapy is appropriate for you as part of a comprehensive treatment plan. Looking to inquire further about how long does stem cell therapy last for knees, contact us at Stemedix today.

by admin | Jun 14, 2023 | Stem Cell Therapy, COPD, Regenerative Medicine
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease that causes obstructed airflow from the lungs. Affecting an estimated 15 million people in the United States alone, COPD is characterized by progressively worsening symptoms, including breathing difficulty, cough, mucus (sputum) production, and wheezing, and is most often the result of prolonged exposure to cigarette smoke.
Not just an issue for those in the U.S., COPD has been demonstrated to be a preventable and treatable global health challenge. With an estimated 3.5 million worldwide deaths attributed to COPD each year, the disease is currently the third leading cause of death.
While there have been medical advances in the treatment of COPD, these therapies focus primarily on symptomatic relief and not the reversal of lung function deterioration or improvement in patients’ quality of life.
Since stem cells are known to differentiate into a wide variety of cell types and have been previously used to regenerate lung parenchyma and airway structure, they are believed to be an evolving and promising therapeutic treatment option for those with COPD.
Supported by extensive studies exploring the mechanism of stem cells in the regulation of COPD, experts have demonstrated that stem cells possess multidirectional differentiation potential and are able to differentiate into specific forms of alveolar epithelial cells (type I and/or type II) and participate into the repair of lung tissue structure.
In this review, Chen et al. summarize the most relevant findings of eight clinical trials that explore the treatment of COPD with mesenchymal stem cells (MSCs).
These clinical trials, conducted between the years of 2009 – 2020, examined using different modes and doses of a variety of autologous or allogeneic MSCs, including bone marrow-derived stem cells (BM-MSCs), adipose tissue-derived stem cells (AD-MSCs), and umbilical cord-derived stem cells (UC-MSCs), in the treatment of COPD.
Examining the different types of MSCs used for these clinical trials, the authors conclude that while all types of MSCs have benefits in this application, AD-MSCs and UC-MSCs are very promising, primarily because the source is easily available; additionally, the process of collecting UC-MSCs is non-invasive. Looking at trends in recent clinical trials, the authors find a general increase in the shift toward using AD-MSCS and UC-MSCs and away from BM-MSCs, primarily for the reasons mentioned previously.
Analyzing results of these clinical trials related to mode, schedule, and dosage of administration, the authors found that stem cells administered intravenously into the body concentrated in the lungs for thirty minutes before gradually migrating to the liver; the inability of stem cells to keep stem cells in the lungs for a longer period of time was noted as a potential barrier that could limit the effectiveness of stem cell therapy for this condition.
To address this concern, the authors recommend adjusting the schedule and/or mode of administration, indicating that prior research suggests multiple doses and administration via airway injection using a bronchoscope is a good way to deliver stem cells directly to the lungs.
Chen et al. found that regardless of what type of MSCs and what mode of administration was used, stem cell therapy for the management of COPD has been proven to be safe and without evidence of any adverse events. However, only 2 of the eight clinical trials evaluated for this review demonstrated that MSCs could improve pulmonary function. The results of the other six indicated that MSCs had no effect on pulmonary function.
Considering these findings, and in view of the small number of patients in the two clinical trials demonstrating therapeutic improvement on pulmonary function, the authors call for further research to better understand the effects of MSCs on improvements of pulmonary function.
In closing, Chen et al. indicate that stem cell therapy may have a significant role in the future treatment of COPD and other respiratory diseases and offer a number of suggestions for future clinical trials. The recommendations provided by the authors for future clinical trials examining the therapeutic effects of MSCs when treating COPD include expanding the sample size, extending the follow-up time to a minimum of 2 years, selecting patients with different grades of COPD, considering using AD-MSCs and UC-MSCs (rather than BM-MSCs); and further exploring the effects of MSC on change in other inflammatory, immune, and metabolic indicators.
Source: “Stem cell therapy for chronic obstructive pulmonary disease – PMC.” 15 Jun. 2021, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8280064/.


 St. Petersburg, Florida
St. Petersburg, Florida
