
by admin | Mar 6, 2025 | Stem Cell Research, Stem Cell Therapy, Umbilical Stem Cell
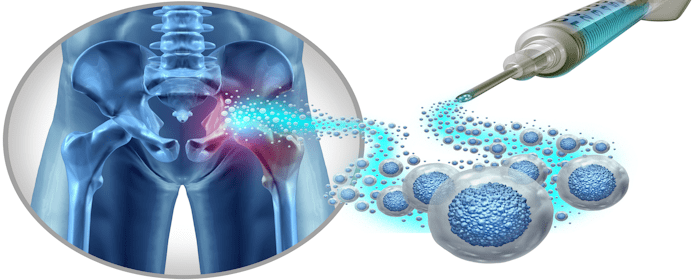
Osteonecrosis of the femoral head (ONFH) is a serious condition that affects the hip joint, leading to bone damage and joint problems. The disease occurs when the blood supply to the femoral head (the top part of the thigh bone) is disrupted, leading to small fractures and a failure of the bone to repair itself.
ONFH is a significant health issue worldwide. In the United States, approximately 20,000 to 30,000 people are diagnosed with ONFH each year. In China, more than 8 million individuals over the age of 15 suffer from nontraumatic ONFH annually. This condition mainly affects younger and middle-aged adults, making long-term treatment outcomes particularly challenging.
One of the most common treatment options for severe ONFH is total hip arthroplasty (THA), also known as hip replacement. However, THA has limitations, including a high revision rate and a limited lifespan for the artificial joint.
To preserve the natural joint and delay or avoid surgery, early intervention is essential. Several treatments are currently available, including medication, physical therapy, and surgical procedures like core decompression and bone grafting. However, these methods produce inconsistent results, meaning that better treatment options are still needed.
One promising approach involves mesenchymal stem cell (MSC) therapy. MSCs play an important role in bone healing, and their use in treating ONFH has been studied extensively.
In this study, Zhao et al. explore the available evidence for the therapeutic effect of human umbilical cord mesenchymal stem cells (HUCMSCs) on early-stage traumatic ONFH.
Potential of Stem Cell Therapy in ONFH Treatment
ONFH leads to bone cell death due to lack of blood supply. In patients with ONFH caused by excessive alcohol consumption or steroid use, the ability of MSCs to form new bone is significantly reduced. This results in an imbalance between bone formation and bone loss, leading to the weakening and collapse of the femoral head.
The authors report that adding new MSCs from an external source, such as HUCMSCs, may help by replenishing lost cells and stimulating bone regeneration. Studies have shown that MSCs from healthy individuals can be transplanted into patients without causing immune rejection. MSCs have already been used successfully in regenerating various types of tissues, and they can be obtained from several sources, including bone marrow, fat tissue, and umbilical cords.
BMMSCs are the most commonly studied type of MSCs, but their use is limited because they become less effective with age and disease. Research comparing the effectiveness of different stem cell sources has found that HUCMSCs may be a better alternative. These cells are easily obtained from umbilical cords, involve no ethical concerns, and have strong growth potential. Because of these advantages, HUCMSCs have been proposed as a promising treatment for ONFH.
Safety of Stem Cell Therapy
The authors cite several studies that have analyzed the safety of transplanting both BMMSCs and HUCMSCs. For example, one study following patients for 12 months after receiving MSC therapy found no serious adverse effects. Another study tracked patients for three years and reported no significant side effects.
HUCMSCs, in particular, have been found to improve the local healing environment by secreting factors that reduce inflammation and promote tissue repair. Experimental studies in animals also confirm the safety of HUCMSCs, showing no immune rejection or tumor formation after transplantation.
Effectiveness of HUCMSCs in Treating ONFH
To maximize the effectiveness of HUCMSC therapy, the authors focused on optimizing how the cells are delivered to the femoral head. Intravenous (IV) injection of MSCs demonstrated some benefits, but the number of stem cells that actually reach the affected area was limited. To improve results, researchers also tested direct injection of HUCMSCs into the femoral head, ensuring a higher concentration of cells in the damaged area.
Studies have shown that injected HUCMSCs can survive and function in the low-oxygen and damaged environment of the femoral head. At four weeks after transplantation, a significant number of HUCMSCs were detected in the bone, but by eight weeks, their numbers had decreased. According to the authors, this suggests that the transplanted cells either died or migrated to other areas over time. Despite this, the therapeutic effects at four weeks were better compared to untreated ONFH cases. Imaging studies and tissue analysis confirmed that bones treated with HUCMSCs had improved structure and reduced damage compared to those that did not receive treatment.
Clinical Implications and Future Research
According to Zhao et al., current guidelines suggest that for patients with early-stage ONFH, a combination of core decompression and MSC therapy may be beneficial. Research has shown that MSCs work best when provided in a low-oxygen environment, which enhances their ability to regenerate bone. Further studies are needed to refine MSC treatment strategies, determine the best dosage, and evaluate long-term outcomes.
Future research should also explore ways to prolong the survival of transplanted MSCs in the femoral head. One potential approach is preconditioning MSCs with low oxygen before transplantation to enhance their ability to function in damaged tissue. Other studies suggest that combining MSC therapy with additional bone-supporting treatments, such as growth factors or specialized scaffolds, may improve outcomes.
Stem Cell Therapy for ONFH: A Promising Approach
The authors conclude that HUCMSC therapy offers a promising new approach to treating ONFH by replenishing damaged bone cells, improving blood supply, and reducing inflammation. Compared to other types of stem cells, HUCMSCs have advantages such as easy availability, strong regenerative potential, and low risk of immune rejection. While safety concerns remain, current studies indicate that HUCMSCs are well tolerated and do not cause severe side effects.
Despite this promising approach, ongoing research will help refine the use of HUCMSCs for ONFH treatment and determine the most effective ways to enhance their therapeutic potential. With further development, HUCMSC therapy may become a standard option for preserving hip joint function and delaying or preventing the need for hip replacement surgery.
Source: Zhao J, Meng H, Liao S, Su Y, Guo L, Wang A, Xu W, Zhou H, Peng J. Therapeutic effect of human umbilical cord mesenchymal stem cells in early traumatic osteonecrosis of the femoral head. J Orthop Translat. 2022 Oct 14;37:126-142. doi: 10.1016/j.jot.2022.09.008. PMID: 36313533; PMCID: PMC9582590.

by admin | May 3, 2023 | Stem Cell Therapy, COPD, Mesenchymal Stem Cells, Stem Cell Research, Umbilical Stem Cell
Chronic obstructive pulmonary disease (COPD) is an incurable and debilitating disease characterized by chronic and progressive inflammation that leads to small airway obstruction and emphysema.
According to the World Health Organization, COPD is the third leading cause of death and is responsible for an estimated 3.2 million deaths each year. Between 80 and 90% of all COPD cases are caused by exposure to cigarette smoke, meaning it is also one of the most preventable diseases.
In addition to the increased risk of death, COPD significantly affects the overall quality of life and is often associated with difficulty breathing, chronic cough, lack of energy, lung infections, lung cancer, and heart disease.
A number of stem-cell-based approaches to address this issue are currently being explored. In this study, Ridzuan et al. uses an animal model to assess the potential anti-inflammatory effects of human umbilical cord mesenchymal stem cell (hUC-MSC)-derived extracellular vesicles (EVs) in cases of COPD.
EVs are small membrane vesicles of multivesicular bodies that are released by a variety of cells, including MSCs. Studies have demonstrated EVs isolated from MSCs mimic the therapeutic effects of MSCs.
Over the course of this study, and to mimic the symptoms observed with COPD, rats were exposed to cigarette smoke for up to 12 weeks, followed by transplantation of hUC-MSCs or application of hUC-MSC-derived EVs.
At the conclusion of this study, Ridzuan et al. found that both the transplantation of hUC-MSCs and the application of hUC-MSC-derived EVs reduced peribronchial and perivascular inflammation, slowed alveolar septal thickening, and decreased the number of goblet cells. Both applications also improved the loss of alveolar septa in the lung of COPD rats and regulated multiple pathways commonly associated with COPD.
Ridzuan et al. conclude that hUC-MSC-derived EVs effectively reduce COPD-induced inflammation and could have the potential to be a therapy for the management of COPD.
The authors also concluded that the selected treatment methods decreased the above-described symptoms at comparable rates. While there are still limited data demonstrating the regenerative and the anti-inflammatory effects of MSC-EVs to mitigate the inflammation in COPD, further study is needed to fully understand the anti-inflammatory effects of MSC-EVs and to better understand the specific mechanisms of action of all contents of MSC-EVs as they relate to the potential future treatment of COPD.
Source: “Human umbilical cord mesenchymal stem cell-derived extracellular ….” 12 Jan. 2021, https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-02088-6.

by admin | Jan 5, 2023 | Mesenchymal Stem Cells, Diabetes, Regenerative Medicine, Stem Cell Research, Stem Cell Therapy, Umbilical Stem Cell, Wharton's Jelly
Mesenchymal stem cells (MSCs) are multipotent fibroblast-like cells found throughout the body and have been found to have self-renewing and multilinear therapeutic potential by providing new cells for tissue repair by replacing damaged cells.
Thought to stimulate repair and control the immune response through an expression of growth factors and other cytokines, MSCs are at low risk of rejection and repair tissue damage through immunomodulation, not by their ability to differentiate.
While MSCs can be isolated from a number of tissue sources, including bone marrow, peripheral blood, adipose (fat) tissue, umbilical cord blood, and umbilical cord tissue (Wharton’s jelly). MSCs derived from the human umbilical cords (UCMSCs) have been found to have significant advantages over MSCs isolated from other sources. These advantages include higher proliferation and self-renewal capacity and multilineage differentiation capability.
Unlike many sources of MSCs, the umbilical cord is considered medical waste, making the collection of UCMSCs noninvasive and eliminating ethical concerns associated with the collection of MSCs from other sources. These UCMSCs have been developed as effective “off-the-shelf” cell therapy for a number of conditions, including autoimmune diseases, and as a treatment for a number of emergency medical conditions.
This Phase 1 clinical study, designed and conducted by Chin et al., intended to determine the safety and efficacy of intravenous allogeneic infusion of UCMSCs in healthy volunteers and to determine the effective dose at which an immunomodulatory effect is observed. The findings of this study are intended to serve as a guideline and benchmark for future CVL-100 clinical research.
Analyzing the results of this clinical study, the authors report that there was no observed complication resulting from the infusion and no significant adverse event in either dosage group in the 6 months of follow-up. These findings led Chin et al. to conclude that UCMSCs infusion was safe among healthy subjects, results that were consistent with other UCMSC treatment-based studies.
The authors also reported that UCMSCs infusion posed no significant adverse effects in patients with type 2 diabetes. Despite the relatively small sample group of this study (11 subjects), the authors reported demonstrating an initial transient proinflammatory effect followed by a significant and prolonged anti-inflammatory effect.
In addition, Chin et al. report found that high-dose (HD) CLV-1000 infusion provided a significant increase in both hemoglobin level and MCV level that falls within the normal range. Biomarker assessment results also indicated that the HD group demonstrated a significant steady increase of cytokine IL-1RA from baseline up until 6 months of posttreatment. This finding is especially important as IL-1RA is a naturally occurring antagonist to the proinflammatory cytokine 1L-1.
The authors conclude that this study clearly demonstrates a difference in immunomodulatory effect between the high-dose and low-dose treatment groups, with the HD group demonstrating a significantly greater reduction of proinflammatory cytokine TNF-α and an increased level of specific anti-inflammatory cytokines within 6 months and in relation to those in the low dose group. Considering this, Chin et al. conclude that a CLV-100 dosage of two million MSCs per kilogram of body weight represents the optimal dose level for overcoming inflammatory conditions by displaying the best improvement in all parameters tested, absence of side effects, and SAEs.
The data collected in this study also suggests that this is the first study to report a significant reduction of globulin observed over the course of the study. This is important because globulin serves an important role in immunity. Additionally, increases in serum globulins are associated with several immune-mediated diseases, including rheumatoid arthritis, chronic liver disease, diabetes mellitus, and cancer.
Considering these findings, the authors of this study conclude that high doses of allogeneic MSCs could help exert beneficial effects of repair and healing.
Source: “High Dose of Intravenous Allogeneic Umbilical Cord-Derived ….” https://www.hindawi.com/journals/sci/2020/8877003/.

by admin | Jul 12, 2019 | Age Management, Exosomes, Stem Cell Therapy, Umbilical Stem Cell
As we age, the appearance and structure of our skin changes. This is plain for all to see—we can usually estimate a person’s age simply by looking at their skin. Young skin is full of molecules that keep it thick, plump, and supple, such as collagen and elastin. Over time, the skin produces less and less of the substances. Consequently, aging skin is thinner and it loses its strength and elasticity. As such, the skin develops fine lines and deep wrinkles. It also becomes lax and begins to sag.
Scientists know that the quantity of collagen, elastin, and other proteins make the difference between young and old skin. Not surprisingly, doctors have been trying for decades to increase the levels of these molecules in the skin in an effort to reverse the signs of aging skin. Some approaches work for short periods of time. For example, laser and intense pulsed light treatments can stimulate the skin to produce these youthful molecules. Another approach is to directly inject collagen and other substances into the skin. The Holy Grail of skin rejuvenation, however, is to find a way to make the skin naturally produce more of these substances. Recent research suggests that stem cells could be the answer.
Researchers collected mesenchymal stem cells from umbilical cords. This tissue is removed and discarded after a woman gives birth to a baby. The scientists then collect the tiny sacs called exosomes from these umbilical cord stem cells. Exosomes are densely packed with proteins, RNA, and other important molecules that are important for growth in rejuvenation. The researchers then simply applied these exosomes to samples of human skin to see if they could influence skin rejuvenation.
The first remarkable finding of this research was that exosomes taken from umbilical cord mesenchymal stem cells were absorbed into human skin. Why is this important? Because it means that if exosomes are used as a potential treatment, they can be placed on the skin rather than injected into the skin. The second remarkable finding is that exosomes, once they cross into the skin, are taken up by skin cells (human dermal fibroblasts). Once inside the skin cells, the exosomes take over the cells, in a way. They prompt the cells to produce more collagen and elastin than normal. The exosome-treated skin cells also attract other cells to the skin. We know from other work that more collagen, more elastin, and more cells within the skin leads to plumper, fuller, more elastic skin.
While clinical trials are needed to confirm this research, this work strongly suggests that the exosomes from umbilical cord-derived mesenchymal stem cells have the ability to rejuvenate human skin. Perhaps most impressively, these potential skin rejuvenating exosomes can be applied topically, such as within a cream or ointment. Thus, patients could receive the potential benefits of this treatment, while avoiding painful injections. Again, more work needs to be done before this research becomes a routine treatment, but the results are quite promising.
Reference: Kim, YJ. et al. (2017). Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate rejuvenation of human skin. Biochemical and Biophysical Research Communications. 2017 Nov 18;493(2):1102-1108.

by admin | Jun 27, 2019 | Stem Cell Therapy, Umbilical Stem Cell
Psoriatic arthritis is a chronic inflammatory condition that can become profoundly disabling. As a form of arthritis, the condition causes swollen, painful joints. The number and types of joints affected can vary over time, but most patients have polyarthritis i.e. arthritis in more than one body joint. Psoriatic arthritis also causes debilitating inflammation of the tendons, typically in the hands. Making matters worse, patients with psoriatic arthritis also suffer from skin rashes, eye problems, kidney and gastrointestinal problems, and profound fatigue. About one in five patients with psoriatic arthritis eventually develop severe manifestations of the disease in which the joints become permanently deformed and the surrounding bone breaks down.
The treatment of psoriatic arthritis usually includes a combination of drug and non-drug treatments. Nondrug treatments for psoriatic arthritis include exercise, physical therapy, and weight loss. Mild psoriatic arthritis is usually treated with nonsteroidal anti-inflammatory drugs such as naproxen. Moderate to severe psoriatic arthritis generally requires disease modifying anti-rheumatic drugs (DMARDs), which can include biologic and non-biologic agents. The typical non-biologic treatment for psoriatic arthritis is methotrexate. With the exception of severe disease, most physicians try methotrexate before using a biologic DMARD. If methotrexate fails, patients usually must move to one of the biologic agents, antibodies that are injected under the skin. Unfortunately, all DMARDs are associated with certain and sometimes severe side effects, and not every DMARD works for every patient with psoriatic arthritis.
Because psoriatic arthritis is potentially disabling and often difficult to treat effectively, researchers are aggressively pursuing other treatments. Stem cells offer a unique opportunity to provide patients with cells that can regenerate damaged joints and reverse the signs and symptoms of arthritis. To this end, Margaret Coutts and colleagues harvested stem cells from umbilical cord samples—the tissue that is routinely corrected after newborns are delivered is usually discarded as medical waste. They selected a patient with severe psoriatic arthritis would fail to find relief after nonsteroidal anti-inflammatory drugs, methotrexate, and biologic DMARDs. The researchers purified stem cells from cord blood and administered 200,000 cells per day to the 56-year-old man over a period of five days.
Within one week of umbilical cord stem cell treatment, the patient reported fewer and less severe psoriatic skin plaques and less joint pain. Encouraged by these results, the psoriatic arthritis patient continued receiving three rounds of stem cell treatments over six months. At the end of these treatments, the psoriatic skin plaques were almost completely gone, and the ones that remained were smaller, less prominent, and lost their red, scaly appearance. The man had substantially less joint pain and swelling and reported feeling “higher energy levels” and greater physical functioning. Lastly, laboratory markers of inflammation including ESR and CRP were noticeably improved.
Since these dramatic improvements occurred for only one person, they should be evaluated with caution. Additional studies with larger numbers of people are needed to make definitive conclusions. Nevertheless, umbilical cord stem cells led to profound improvements in this psoriatic arthritis patient’s life, a result that cannot be overstated.
Reference: Coutts, M. et al. (2017). Umbilical cord blood stem cell treatment for a patient with psoriatic arthritis. World Journal of Stem Cells. 2017 Dec 26; 9(12): 235–240.

by admin | Jun 14, 2019 | Mesenchymal Stem Cells, Umbilical Stem Cell
Mesenchymal stem cells are believed by many to be the most effective type of stem cell for regenerative medicine. Mesenchymal stem cells are intriguing because they can regenerate damaged tissues in four major ways:
Paracrine effects – Mesenchymal stem cells release substances that can attract other cells to the site of injury. For example, mesenchymal stem cells secrete cytokines to attract cells that participate in wound healing.
Trophic effects – Mesenchymal stem cells release substances that increase blood vessel development and help cells grow and survive.
Immunomodulation – Mesenchymal stem cells have anti-inflammatory properties, exerting beneficial effects in multiple sclerosis, graft versus host disease, Crohn’s disease, ulcerative colitis, and lupus, among others.
Differentiation – Since they are pluripotent, mesenchymal stem cells have the potential to become other cells such as bone cells, fat cells, brain cells, skin cells, blood vessel cells, and many others.
Unfortunately, it can be difficult to collect mesenchymal stem cells. One major source of mesenchymal stem cells is bone marrow. To collect bone marrow mesenchymal stem cells, however, a person (usually the patient) must undergo a procedure to obtain bone marrow. This procedure is invasive and can be uncomfortable. Therefore, researchers are keenly interested in finding other sources of mesenchymal stem cells.
One very attractive source of mesenchymal stem cells is the umbilical cord. For centuries, umbilical cord tissue was considered medical waste. Once a baby was born and the umbilical cord was cut, the rest of the umbilical cord was usually discarded. Approximately 30 years ago, however, researchers discovered that umbilical cords that were destined to be destroyed as medical waste actually contained cells that could be medically useful. Fifteen years ago, researchers showed that cells taken from umbilical cords contained mesenchymal stem cells that have the ability to become other cells (e.g. fat or bone cells).
Since 2004, researchers have discovered an incredible number of potential uses for mesenchymal stem cells that come from umbilical cord tissue. In fact, research shows that mesenchymal stem cells are taken from discarded umbilical cord actually have higher levels of certain helpful genes then mesenchymal stem cells taken from fat tissue, bone marrow, or skin. Perhaps most impressively, umbilical cord mesenchymal stem cells are non-tumorigenic, which means they do not produce tumors.
Today, mesenchymal stem cells derived from the umbilical cord are the subject of intense clinical research. There are approximately 100 clinical trials testing the safety and effects of umbilical cord mesenchymal stem cells in over a dozen different diseases. In all clinical studies, these stem cells have proven to be remarkably safe—there have been no side effects reported aside from a temporary fever in some cases.
Taken together, these results suggest human umbilical cord is an excellent source of mesenchymal stem cells for several reasons. Unlike embryonic stem cells, there are no ethical problems collecting umbilical cord tissue for stem cells. These particular stem cells appear to be a bridge between prenatal and postnatal mesenchymal stem cells and possess the beneficial properties of each. They do not form tumors, but they do grow in number and become adult cells. As such, human umbilical cord mesenchymal stem cells are unique and are a promising resource in regenerative medicine.
Reference: Arutyunyan, I. et al. (2017 Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells International. 2016:6901286.




 St. Petersburg, Florida
St. Petersburg, Florida
