
by admin | Jun 24, 2022 | Mesenchymal Stem Cells, Exosomes, Stem Cell Research, Stem Cell Therapy
Mesenchymal stem cells (MSCs) have been widely studied and increasingly recognized as a potential therapeutic with the ability to initiate and support tissue regeneration and remodeling. While over 1100 clinical trials have been conducted to assess the therapeutic benefits of MSCs, there continues to be widespread variation surrounding the potential treatment outcomes associated with these cells.
This review, authored by Chang, Yan, Yao, Zhang, Li, and Mao, focuses primarily on profiling the effects of the secretome, or the effects of paracrine signals of MSC, as well as highlights the various engineering approaches used to improve these MSC secretomes. Chang et al. also review recent advances in biomaterials-based therapeutic strategies for the delivery of MSCs and MSC-derived secretomes.
Recent research has demonstrated paracrine signaling as the primary mechanism of MSC therapeutic efficacy. This shift towards the MSC secretome in applications ranging from cartilage regeneration to cardiovascular and other microenvironments has demonstrated its therapeutic potential in prevalent injury models. Additionally, the versatility of MSCs allows them to be specifically tailored using biomaterials toward specific therapeutic outcomes.
A specific example of MSC secretome’s therapeutic potential is their ability to support cardiovascular tissue repair through minimization of fibrotic scarring of cardiac tissue typically observed to occur during a myocardial infarction (MI). Additionally, research has demonstrated MSC secretomes facilitate the proliferative, angiogenic, and anti-inflammatory phases of the wound healing process.
Secretome transfer occurring between MSCs and other cells in the target area primarily occurs through the release of extracellular vesicles (EVs) and is considered a safer form of therapeutic application compared to MSC therapy. MSC secretomes can also be specifically engineered through hypoxia, treatment with bioactive agents, and modulating cell-cell and ECM interactions in the MSC culture.
One of the biggest challenges facing the therapeutic efficacy of MSC is their limited cell survival, retention, and engraftment following injection or transplantation (found to be as low as 1% surviving one day after implantation). Recent studies have demonstrated MSC secretome, and specifically, EVs, although they remain a significant obstacle, are a promising alternative and able to bypass a number of cellular challenges, including cell survival.
Further consideration and approaches to increasing survival rates of MSCs include experimenting with a wide variety of biomaterials as a way to promote adaptation in the target implantation area. This includes looking for biomaterials to regulate oxygen tension levels, glucose supply, mechanical stress, and pH levels, which collectively can be used to regulate metabolic pathways of the MSC, effectively influencing cell survival and their ability to be used as therapeutic treatment options.
Despite the recent advances in the use of MSC secretomes and their delivery strategies, Chang et al. call for continued study of the subject and specifically recommend developing a specific set of paracrine cues to be used as a well-defined formulation in future therapeutic applications.
The authors also point out that the use of EVs and other direct applications of the MSC secretome are thought to be promising for the treatment of osteoarthritis, ischemic stroke, and coronavirus-related diseases. Considering this, Chang et al. highlight the increasing need to fully understand the paracrine signaling effects of MSC therapies and the delivery strategies associated with this application.
Source: “Effects of Mesenchymal Stem Cell‐Derived Paracrine Signals and ….” 12 Jan. 2021, https://onlinelibrary.wiley.com/doi/full/10.1002/adhm.202001689.

by Stemedix | Jun 20, 2022 | Transverse Myelitis, Stem Cell Research, Stem Cell Therapy
Patients suffering from uncommon conditions, such as transverse myelitis, often have limited treatment options, since these conditions lack the funding and the research needed to understand the disease and its causes. Here we will discuss stem cells for Transverse Myelitis.
Regenerative medicine, also known as stem cell therapy, unlocks a new understanding of conditions and treatments. Physicians are discovering the potential of new methodologies, such as stem cell therapy. This alternative therapy may offer options for rare and common conditions affecting millions of patients worldwide.
Transverse myelitis is a rare condition, affecting only about 1400 people per year. Understanding and treating transverse myelitis can improve the lives of patients and their loved ones and may lead to breakthroughs in treating related disorders and diseases with overlapping symptoms.
What Is Transverse Myelitis?
Transverse myelitis is a neurological condition in which inflammation in the spinal cord damages the protective coating around the neurons, called myelin. Damaged myelin disrupts communication between the nerves in the spinal cord and the rest of the body, which can result in partial or total paralysis.
Patients with transverse myelitis often experience weakness in their arms and legs, bladder and bowel dysfunctions, sharp, radiating pain, and abnormal sensations like burning, tingling, and numbness.
How Does Stem Cell Therapy Work?
Stem cells can differentiate into different types of specialized cells that develop into blood, bones, organs, and other tissues. These specialized cells can repair, restore, replace, and regenerate cells.
In stem cell therapy treatments, physicians strategically administer the cells to best target damaged or destroyed tissues. The stem cells then work to rapidly regenerate, differentiating into the cells necessary to heal the damaged tissue.
How Can Stem Cells Manage Transverse Myelitis?
Researchers are investigating stem cell treatments for transverse myelitis by exploring the potential for stem cells to restore demyelinated spinal cords. These studies help physicians understand how transverse myelitis damages the myelin sheath and the mechanisms that may be able to treat the disease.
Researchers are currently seeing promising results in differentiating stem cells into healthy motor neurons, directing the new cells to repair the damaged myelin sheath and restore their connection with muscle fibers.
Additionally, stem cell therapies show promise in regulating the immune system and regulating inflammatory proteins, working to restore function to the spinal cords of patients who have partial paralysis due to the progression of transverse myelitis.
Stem cell studies working with transverse myelitis patients work to discover new possibilities in preventing the progression of the disease, reducing the relapse rate, and diminishing the harmful inflammatory effects of the condition. If you would like to learn more about stem cells for Transverse Myelitis contact us today!

by Stemedix | Jun 13, 2022 | Neurodegenerative Diseases, Stem Cell Therapy
Regenerative medicine, also known as stem cell therapy, offers a new range of treatment options for patients suffering from neurological disorders. Here we will discuss Stem Cell Therapy for Neurological Disorders.
Neurological conditions often rely on symptom management through medication. However, stem cell therapy has begun to emerge as a new alternative management option for neurological conditions.
How Does Stem Cell Therapy Work?
Stem cells are the only cells in the human body that can differentiate into specialized cells. Stem cells lie dormant throughout the body in bone marrow, fat tissues, and various organs until they’re needed to regenerate lost or damaged tissue.
When stem cells regenerate, they undergo a process called asymmetrical division. In this process, one cell becomes a perfect replica of the stem cell, and the other cell becomes a specialized cell. Stem cells also reproduce rapidly, so they react quickly when called to action.
The new cells work to repair or replace damaged cells, heal wounds, and restore function lost through dead or damaged cells.
What Neurological Disorders Benefit from Stem Cell Therapy?
As regenerative medicine research continues to grow, studies exploring the effects of stem cell therapy on neurological disorders have shown positive results in helping to manage many conditions such as:
Cerebral Palsy
Cerebral palsy is a condition that develops before, during, or shortly after birth due to damage to the brain. Symptoms of cerebral palsy in children include poor coordination and underdeveloped reflexes.
In studies, stem cell therapy shows the potential to replace damaged or nonfunctional brain cells in cerebral palsy patients, provide support to the neurons, and reduce scarring in the brain.
Multiple Sclerosis (MS)
Multiple sclerosis is an autoimmune disease in which the immune system attacks the central nervous system and damages the nerve fibers. The damage to the nervous system interrupts the communication between the brain and the rest of the body, causing symptoms like pain, weakness, and vision loss.
Trials exploring stem cell therapy in treating MS resulted in most patients not experiencing a relapse in MS symptoms or brain lesions for five years after their treatment.
Parkinson’s Disease
Parkinson’s disease is a neurodegenerative condition in which the loss of neurons in an area of the brain stem reduces dopamine production. As a result, patients with Parkinson’s disease can experience problems with movement, muscle control, gait, and balance.
In early studies, stem cell therapies worked to replace the lost neurons, and patients saw a reduction in muscle rigidity and tremors.
While there’s plenty of work needed to understand the most effective methodology and course of treatment, early research on using regenerative medicine to treat neurological disorders points to promising results. If you would like to learn more about stem cell therapy for Neurological disorders, contact a care coordinator today at Stemedix!

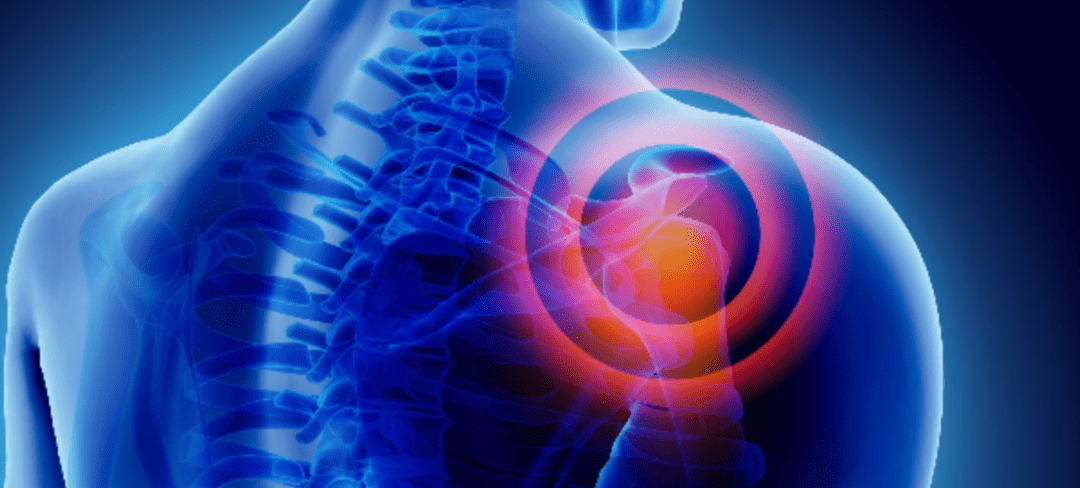
by Stemedix | May 30, 2022 | Pain Management, Athletic Injury, Stem Cell Therapy
The human shoulder is not as simple as it looks from the outside. It’s made of multiple bones, tendons, and muscles that all work together to give you a full range of motion. The three bones in the shoulder are the scapula (shoulder blade), the humerus (upper arm bone), and the clavicle (collar bone). In this article with will discuss shoulder impingement syndrome.
A group of tightly packed muscles known as the rotator cuff stretches from your shoulder blade to the top of your humerus to keep the humerus sitting comfortably in the glenohumeral joint, or shoulder joint. The rotator cuff is what gives you the ability to rotate your arms and raise them above your head.
However, with so many moving parts packed into such a small area, there are lots of opportunities for something to go wrong. Since the rotator cuff sits between two bones, it’s vulnerable to becoming pinched between them. This is known as shoulder impingement syndrome.
What Causes Shoulder Impingement Syndrome?
Shoulder impingement syndrome can be caused by anatomical abnormalities, such as bone spurs, that limit the amount of room the humerus has to move within the shoulder joint. However, it’s more often caused by overuse of the shoulder or injury.
When the rotator cuff is overused, injured, or irritated, the tendons begin to swell. You’ve probably experienced swelling in other parts of your body before. It’s uncomfortable, but it’s usually not a big deal and subsides within a few days. But since the rotator cuff is surrounded by bone, it doesn’t have room to swell without the tendons rubbing against bone.
The more the tendons rub against bone, the more swollen they become. And the more swollen they become, the more they rub against the adjacent bones. It’s a vicious circle that can be hard to break.
How To Manage The Pain
Shoulder impingement syndrome can limit your range of motion by causing weakness and stiffness in your arm and making it painful to lift, reach, and rotate your arm. But the pain can be managed using a few different methods.
When the syndrome is caught early, physical therapy can be very effective at reducing inflammation, improving your range of motion, and strengthening your rotator cuff. NSAIDs like ibuprofen, aspirin, and naproxen can also be taken to temporarily reduce the pain caused by swelling and inflammation. For severe cases of shoulder impingement syndrome, surgical intervention may be required.
However, an increasing number of people are looking into regenerative medicine as an alternative option to avoid surgery and, in some unavoidable cases, recover from surgery. Mesenchymal stem cells offer a potential therapeutic and restorative option to help manage pain, decrease inflammation, and repair damaged tissues. Their paracrine signaling through extracellular vesicles generates a regenerative microenvironment that helps to inhibit scar tissue formation, reduce inflammation, and promote angiogenesis. If you would like to learn more about the treatment options for shoulder impingement syndrome, contact a care coordinator today at Stemedix!

by Stemedix | May 23, 2022 | Degenerative Disc Disease, Stem Cell Therapy
Back pain is one of the most frequent physical complaints among adults. It’s also the leading cause of missed work in the U.S. Most back pain goes away on its own within a week or two, but if you have persistent severe back pain or pain that is accompanied by other symptoms, it may be time to see a specialist. Here we will learn when to see a specialist about back pain.
Signs Your Back Pain May Require Treatment
Rest, ice packs, and mild stretching are often enough to relieve back pain, but pain is the body’s way of telling you something is wrong. If you’re experiencing any of the following along with your back pain, make an appointment with a healthcare professional.
Numbness and Tingling
Numbness, burning, tingling, or pain that radiates into the buttocks and down one or both legs could be a symptom of spinal stenosis.
Spinal stenosis is an orthopedic condition that causes the spinal canal to narrow and put pressure on the spinal nerves. Treatments for spinal stenosis include physical therapy, pain medication, surgery, and non-invasive regenerative medicine.
Incontinence
Experiencing urinary or bowel incontinence with back pain is a sign of a serious problem known as cauda equina syndrome. With this condition, the compression of a grouping of nerves — the cauda equina nerves — causes a loss of bowel or bladder control. Seek emergency medical help if you experience incontinence with back pain.
High Fever
A fever may indicate that your back pain is caused by an infection. Fever is a reaction of the immune system as it tries to combat harmful germs before they do severe damage.
People with compromised immune systems or autoimmune diseases may have difficulty fighting off infection naturally. It’s possible that the two symptoms are unrelated, but fever with back pain could indicate an infection of the spine, a bladder or kidney infection, spinal cancer, shingles, or other conditions that require medical intervention.
Back Trauma
If you have taken a fall, been in a car accident, or experienced another type of trauma, your back pain could be caused by an undetected back injury.
It’s normal to feel stiff or sore for the first 24-72 hours after a trauma, but if rest and over-the-counter pain medication aren’t enough to reduce your pain, you may have injured a muscle or have a small fracture in one of the bones in your back.
Don’t Ignore Pain
Don’t assume your back pain is “nothing” or that it will go away on its own. Back problems can turn into serious health issues if they’re not taken care of properly. If you have any of the above symptoms or pain that persists even though you’re taking care of yourself, pain management can ease your suffering. If you would like to learn more about pain management for back pain contact stemedix today and speak with a care coordinator.

by admin | May 20, 2022 | Stem Cell Therapy, Mesenchymal Stem Cells
Every year, stem cell therapy gains massive traction due to its incredible regenerative and auto-repair properties. More specifically, patients who deal with chronic, incurable conditions such as multiple sclerosis (MS) are closely following any news about this cutting-edge technology.
What is a stem cell?
A stem cell is a special biological entity that has unlimited differentiation potentials and can become any type of cell, hence is also called an undifferentiated cell. The body keeps a large number of these cells in different sites (e.g. bone marrow, umbilical cord, adipose tissue) in case it endures lesions that need regenerative capacities.
The fascinating feature of stem cells is their ability to differentiate into different cell types, including hepatocytes, nerve fibers, osteocytes, chondrocytes, and keratinocytes.
Are stem cells extracted from fetuses?
Perhaps the unethical aspect of stem cell therapy is the most commonly believed misconception out there. This is because early research focused on extracting stem cells from fetuses and embryos, which is what stuck with media outlets and the general population.
However, as mentioned earlier, stem cells are kept in the body to repair inflicted damage, allowing medical professionals to extract these cells and use them to manage a variety of conditions and their symptoms.
How do stem cells help with MS?
Multiple sclerosis is a chronic condition that’s caused by a type IV hypersensitivity reaction, which occurs when the immune system releases antibodies and specific cells to target a certain tissue. In the case of MS, the immune system attacks the myelin sheaths on nerve fibers that allow for fast bioelectrical transmissions of signals.
Stem cell therapy can potentially help MS progression and symptoms in two major ways:
Immunomodulating
By getting rid of the hyperactive immune cells and replacing them with new regulated ones, using stem cell therapy, the reaction against nerve fibers is potentially halted and symptoms may start to temper down.
Re-myelinization
Instead of targeting the immune system, stem cell therapy also helps by having the ability to regenerate myelin sheath. Note that the process of re-myelinization does not occur spontaneously without having progenitor cells to rely on.
In other words, if the patient does not receive stem cell therapy, the myelin sheaths that were destroyed in the relapse phase are irreversibly lost.
How long does it take for possible symptom improvement?
Typically, patients experience symptom improvement after several months of receiving therapy, with peaking results between the 3rd and 6th-month post-procedure. Some may experience feeling improvements earlier. The types of symptoms expected to improve include all signs that were triggered by multiple sclerosis-related inflammatory and immune reactions.
Is stem cell therapy superior to conventional treatment?
The answer to this question is not straightforward, as many factors fall into play. To keep it short, conventional therapy focuses on suppressing your immune system, which predisposes you to several infectious pathogens. Moreover, it cannot modulate the immune system nor regenerate the damage inflicted on the nerve fibers.
Incorporating stem cell therapy in the treatment of MS has opened a door to new opportunities to manage a condition that was initially thought incurable. It is important to remember that this is a management tool that can be done in conjunction with traditional medicine as well as healthy lifestyle choices.



 St. Petersburg, Florida
St. Petersburg, Florida
