
by Shoot To Thrill Media | Oct 22, 2025 | Inflammatory Bowel Disease
Medical Review: Dr. Gerald Mastaw, MD – Board-Certified Physician
Last Updated: October 2025
Understanding IBD
Inflammatory Bowel Disease (IBD) is a chronic inflammatory condition of the digestive tract.
The two primary types are:
- Crohn’s disease: Inflammation can appear anywhere in the gastrointestinal (GI) tract, often in patchy segments.
- Ulcerative colitis: Involves continuous inflammation of the colon and rectum.
Common Symptoms
- Abdominal pain or cramping
- Frequent or urgent bowel movements
- Diarrhea (sometimes with blood)
- Fatigue and weight changes
- Cycles of flares (active inflammation) and remission (calmer periods)
Triggers can vary from person to person, stress, infections, or dietary sensitivities often play a role.
IBD is not the same as IBS.
Unlike irritable bowel syndrome, IBD causes visible inflammation and tissue damage to the intestinal lining.
Diagnosis
IBD is diagnosed through:
- Colonoscopy and biopsy to confirm inflammation
- Imaging (CT, MRI, or capsule studies) to assess the extent of disease
- Stool and blood tests (e.g., calprotectin, CRP) to monitor inflammation and response to therapy
How IBD Is Treated Today
Current management focuses on controlling inflammation, relieving symptoms, and preventing flares.
Conventional Treatment Options
- Medications: Aminosalicylates, corticosteroids, immunomodulators, biologics (anti-TNF, anti-integrin, anti-IL-23), or newer oral JAK inhibitors.
- Lifestyle and nutrition: Gentle diet adjustments during flares, hydration, stress management, and smoking cessation (especially important in Crohn’s).
- Procedures and surgery: Reserved for complications such as strictures, fistulas, or severe disease unresponsive to medication.
- Ongoing monitoring: Colonoscopies and lab work guide treatment and watch for recurrence or precancerous changes.
These therapies can be life-changing, but many patients still experience persistent inflammation or side effects that limit long-term control.
Where Regenerative Medicine May Help
Regenerative medicine seeks to calm the immune system, reduce inflammation, and promote repair of the damaged gut lining, addressing the root cause of IBD rather than just symptoms.
Umbilical Cord Tissue-Derived Mesenchymal Stem Cells (UCT-MSCs)
What they are: Cells isolated from donated umbilical cord tissue after healthy, full-term births.
Why they’re being studied:
- Modulate overactive immune responses
- Release anti-inflammatory growth factors and cytokines
- Encourage the development of regulatory immune cells
- Support repair and regeneration of intestinal tissues
How they’re administered:
- Most studies use IV infusions to deliver MSCs systemically.
- In select Crohn’s cases, cells are placed locally around perianal fistulas to promote healing.
What to expect:
- Protocols vary by trial but generally involve a series of infusions followed by routine monitoring.
- Studies report good tolerability, with minimal adverse effects.
Important:
UCT-MSC therapy for Crohn’s disease or ulcerative colitis is investigational and not FDA-approved.
It is considered a research-based or adjunctive option for patients who have not fully responded to standard treatments.
Recent Clinical Studies on Regenerative Medicine for IBD
2025 – Meta-Analysis on MSC Therapy for Perianal Crohn’s Disease
Title: Efficacy of Mesenchymal Stem Cell-Based Therapies in Perianal Fistulizing Crohn’s Disease: A Systematic Review and Meta-Analysis
Journal: Stem Cell Research & Therapy – Full Text
Summary:
A 2025 review of 25 studies (596 patients) found MSC therapy significantly improved fistula healing rates.
By 6 months, 58% achieved remission (complete closure and no abscess on MRI), a major improvement over placebo.
Benefits persisted up to 12 months, and no serious safety concerns were reported.
Researchers concluded MSC therapy is effective and well-tolerated for refractory perianal Crohn’s disease.
2024 – UC-MSC Infusions for Ulcerative Colitis
Title: Safety and Potential Efficacy of Expanded Umbilical Cord-Derived MSCs in Ulcerative Colitis
Journal: PubMed Central – Full Text
Summary:
Six adults with active UC received three IV infusions of UC-MSCs (100 million cells each).
No serious adverse events occurred over two years of follow-up.
Three of five patients achieved complete clinical remission (Mayo score 0) and discontinued all medications.
The authors concluded UC-MSC therapy may safely induce long-term remission in select ulcerative colitis patients.
2024 – UC-MSC Therapy for Crohn’s Disease Ulcers
Title: Researchers Investigate Use of Umbilical Cord Stem Cells for Crohn’s Disease
Location: Shanghai Clinical Pilot Study – Read Article
Summary:
Seventeen Crohn’s patients unresponsive to other treatments received local and IV UC-MSC therapy.
After 24 weeks, 47% showed major endoscopic improvement and 18% achieved full mucosal healing.
Inflammation markers dropped, and all patients entered clinical remission with no serious adverse effects.
Researchers found UC-MSC therapy helped restore intestinal lining integrity and immune balance.
Could Regenerative Therapy Be an Option for You?
You may wish to explore regenerative medicine if you:
- Have Crohn’s disease or ulcerative colitis that isn’t fully controlled with medications
- Experience frequent flares or medication side effects
- Want an adjunctive option focused on calming inflammation and promoting healing
At Stemedix, we focus on evidence-based regenerative care designed to complement, not replace, conventional treatment.
Our team will review your medical history, current therapies, and goals to determine whether regenerative therapy could fit within your comprehensive IBD management plan.
Medical Disclaimer
This page is for educational purposes only and does not constitute medical advice.
Stem cell therapy for inflammatory bowel disease is not FDA-approved, and individual results may vary.
Always consult a gastroenterologist or qualified healthcare provider before pursuing new treatments.
References
- Zhang L. et al. Efficacy of MSC-Based Therapies in Perianal Crohn’s Disease: A Systematic Review. Stem Cell Res Ther., 2025. Full Text
- Naser R. et al. Expanded UC-Derived MSCs in Ulcerative Colitis. PubMed Central., 2024. Full Text
- Li J. et al. Umbilical Cord Stem Cells for Crohn’s Disease. Shanghai Clinical Pilot., 2024. Full Text

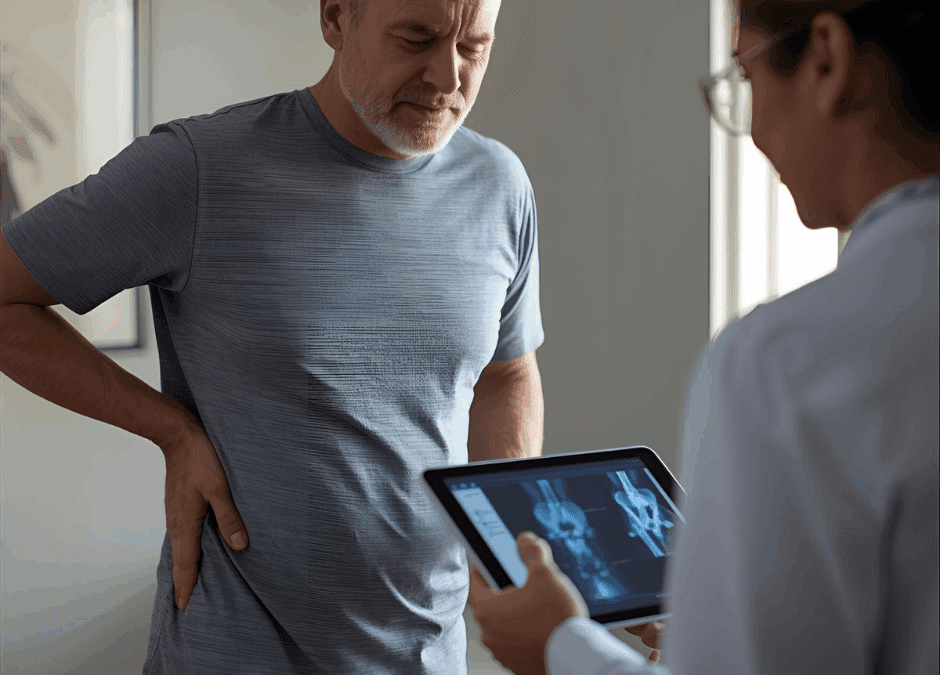
by Shoot To Thrill Media | Oct 22, 2025 | Hip Pain
Medical Review: Dr. Gerald Mastaw, MD – Board-Certified Physician
Last Updated: October 2025
What Is Hip Pain and Why Does It Happen?
The hip joint is one of the body’s largest weight-bearing structures, a ball-and-socket design built to handle movement, rotation, and constant pressure.
When injury, inflammation, or degeneration affects this area, even simple activities such as walking, sitting, or standing can become painful.
Common Causes of Hip Pain
- Osteoarthritis or cartilage wear-and-tear
- Tendonitis or bursitis from overuse
- Hip labral tears or impingement (FAI)
- Muscle or ligament strains
- Post-surgical or traumatic injuries
Typical Symptoms
- Deep aching or stiffness in the groin or outer hip
- Pain that worsens with movement or prolonged sitting
- Clicking, catching, or instability
- Sharp or radiating pain down the thigh
- Decreased flexibility and weakness
Whether the pain is sudden or chronic, hip dysfunction can limit mobility and impact overall quality of life.
Conventional Treatment Approaches
Traditional care focuses on symptom control and preserving joint function.
Standard Therapies May Include:
- Physical therapy, stretching, and low-impact exercise
- Anti-inflammatory or pain-relieving medications
- Corticosteroid injections for short-term relief
- Weight-management or posture correction
- Assistive devices such as canes or braces
For more advanced degeneration, orthopedic procedures may be recommended:
- Arthroscopic repair for labral tears or impingement
- Hip resurfacing or total hip replacement surgery
While these methods can improve symptoms, they often come with downtime, surgical risk, and limited regenerative benefit.
Regenerative Medicine: A Natural Approach to Hip Repair
Regenerative medicine focuses on restoring tissue health and joint function by activating the body’s own repair mechanisms.
For hip-related conditions, the two leading biologic options are:
- Stem Cell Therapy using umbilical-cord-derived or bone-marrow-derived mesenchymal stem cells (MSCs) that may help repair cartilage, tendons, and soft tissue.
- Platelet-Rich Plasma (PRP) a concentrated solution of a patient’s own platelets that release natural growth factors to support healing.
Potential Benefits of Regenerative Therapies
- Decrease inflammation within the joint capsule
- Stimulate repair of cartilage and connective tissue
- Improve mobility, flexibility, and joint stability
- Support pain relief without surgery
- Delay or possibly prevent the need for joint replacement
Note: Stem cell and PRP therapies for hip pain are not yet FDA-approved and remain investigational, but multiple peer-reviewed studies support their safety and therapeutic potential.
Recent Clinical Studies: Regenerative Medicine for Hip Pain
2025 – PRP Outperforms Hyaluronic Acid for Hip Osteoarthritis
Title: Comparative Efficacy of PRP Injection vs. Ultrasound-Guided Hyaluronic Acid Injection in Hip OA Rehabilitation
Journal: PMC – Full Text
Summary:
In this 150-patient study, participants receiving ultrasound-guided PRP injections experienced greater improvements in pain (VAS), stiffness, and function (WOMAC, Harris Hip Scores) than those treated with standard hyaluronic acid.
The PRP group saw statistically significant reductions in pain and disability, confirming PRP’s advantage in managing hip osteoarthritis symptoms.
2024 – Wharton’s Jelly Allograft Reduces Hip OA Pain
Title: Retrospective Evaluation of Cryopreserved Human Umbilical Cord Tissue Allografts in Hip Osteoarthritis
Journal: MDPI – Journal of Clinical Medicine – Full Text
Summary:
Sixty-nine patients with hip osteoarthritis received Wharton’s Jelly (umbilical-cord-derived extracellular matrix) injections.
Within 90 days, pain levels dropped by ~31%; after a second injection, scores improved by ~45%.
Over 78% of patients reported meaningful pain relief, with no major adverse events.
The study concluded that cord-derived biologic injections are safe and may significantly improve comfort and hip function.
2022 – Cord-Derived PRP Provides Faster Relief Than Autologous PRP
Title: Umbilical Cord PRP vs. Autologous PRP for Hip Osteoarthritis
Journal: MDPI – Journal of Clinical Medicine – Full Text
Summary:
One hundred patients with hip OA received either umbilical-cord-derived PRP (C-PRP) or autologous PRP (A-PRP) over three weekly sessions.
Both groups improved, but C-PRP produced faster and greater pain relief at two months and better hip function at one year — especially in early-stage arthritis (Tönnis I–II).
No serious side effects were observed, supporting cord-derived PRP as a safe, effective biologic option for early hip degeneration.
Is Regenerative Care Right for Your Hip Pain?
Regenerative therapy may be a good option if:
- You have mild-to-moderate hip arthritis or injury
- You’ve tried conventional treatments without lasting relief
- You wish to stay active and avoid surgery
- You prefer a natural, biologic approach to joint healing
At Stemedix, our team evaluates your history, imaging, and goals to determine whether PRP or stem-cell-based regenerative therapy could complement your current orthopedic care plan.
Medical Disclaimer
This information is for educational purposes only and should not replace professional medical advice. Stem cell and PRP therapies are investigational and not FDA-approved for hip pain.
Individual outcomes vary. Always consult a licensed medical provider before pursuing any new treatment.
References
- Patel M. et al. PRP vs Hyaluronic Acid in Hip OA Rehabilitation. PMC, 2025. Full Text
- Cazzato G. et al. Wharton’s Jelly Allograft in Hip Osteoarthritis. J Clin Med., 2024. Full Text
- Ricci E. et al. Cord PRP vs Autologous PRP for Hip OA. J Clin Med., 2022. Full Text

by Shoot To Thrill Media | Oct 22, 2025 | Crohn’s Disease
Medical Review: Dr. Gerald Mastaw, MD – Board-Certified Physician
Last Updated: October 2025
What Is Crohn’s Disease?
Crohn’s disease is a chronic inflammatory bowel disease (IBD) that causes ongoing inflammation anywhere along the gastrointestinal (GI) tract — most often the small intestine and the beginning of the large intestine.
This inflammation can lead to pain, fatigue, and poor nutrient absorption.
Common Symptoms
- Abdominal pain and cramping
- Persistent diarrhea or urgent bowel movements
- Unintended weight loss
- Fatigue or low energy
- Fever during flare-ups
- Nutritional deficiencies from poor absorption
Possible Risk Factors
- Family history of IBD
- Overactive or misdirected immune response
- Environmental triggers such as diet, stress, or smoking
Crohn’s symptoms often cycle between flare-ups and remission, which can make long-term management challenging.
Conventional Approaches to Treatment
While there’s currently no cure, standard care focuses on controlling inflammation and preventing complications.
Medications
- Corticosteroids – to quickly reduce inflammation during flare-ups
- Immunosuppressants – to calm the immune system
- Biologic agents – to block inflammatory pathways (e.g., anti-TNF drugs)
Lifestyle & Nutrition
- Identifying and avoiding food triggers
- Eating smaller, more frequent meals
- Staying hydrated and supplementing nutrients as needed
Surgery
- Removing severely damaged intestinal segments
- Treating strictures, abscesses, or fistulas
- Typically reserved for patients who don’t respond to medication
Even with the best care, many patients experience relapses or side effects that affect quality of life — leading some to explore regenerative approaches.
Regenerative Medicine and Crohn’s Disease
Regenerative medicine, particularly using umbilical cord tissue–derived mesenchymal stem cells (UCT-MSCs), is being studied as a novel way to calm inflammation and support intestinal repair.
How MSC Therapy May Help
- Regulates overactive immune responses
- Reduces chronic inflammation in the intestinal wall
- Encourages tissue regeneration and mucosal healing
- Improves nutrient absorption by restoring gut lining integrity
- May lower dependence on steroids or immune-suppressing drugs
Preliminary research suggests that stem-cell-based therapy may lead to:
- Fewer flare-ups
- Improved intestinal healing on imaging and endoscopy
- Enhanced overall well-being and energy
⚠️ Note: MSC therapy for Crohn’s disease is investigational and not FDA-approved. However, clinical evidence continues to expand showing encouraging safety and efficacy results.
Recent Advances in Regenerative Medicine for Crohn’s Disease
2025 – Stem Cell Educator Therapy in Long-Term Crohn’s
Title: Clinical, Immunological, Radiographic, and Pathologic Improvements in a Patient with Long-Standing Crohn’s Disease After Receiving Stem Cell Educator Therapy
Date: July 28, 2025
Link: MDPI – International Journal of Molecular Sciences
Summary:
A 78-year-old patient with chronic Crohn’s received one treatment of Stem Cell Educator therapy using umbilical cord blood stem cells. Within weeks, bowel movements normalized and abdominal pain disappeared. Follow-up colonoscopies at 5 weeks and 6 months showed healed intestinal lining and no active inflammation. Blood tests confirmed major drops in inflammatory markers, and CT imaging revealed full mucosal recovery.
2025 – MSC Therapy for Perianal Fistulizing Crohn’s (Meta-Analysis)
Title: Efficacy of mesenchymal stem cell-based therapies in the treatment of perianal fistulizing Crohn’s disease: a systematic review and meta-analysis
Date: March 28, 2025
Link: Stem Cell Research & Therapy
Summary:
This review of 25 studies involving ~600 patients found that 58% of those treated with MSCs achieved complete fistula closure by 6 months — substantially higher than control groups. No increase in adverse events was noted, confirming strong safety and meaningful clinical improvement in a difficult-to-treat Crohn’s complication.
2024 – Comprehensive Review of Stem Cell Therapy for Crohn’s
Title: Safety and efficacy of stem cell therapy for Crohn’s disease: an umbrella review of systematic reviews
Date: September 30, 2024
Link: PubMed Central
Summary:
Researchers pooled 16 previous reviews of stem-cell-based therapy in Crohn’s disease. Results showed higher remission rates and improved fistula healing compared with conventional treatments. Importantly, MSC therapy did not increase infection or adverse event risks, suggesting it may be a reliable adjunctive treatment in complex cases.
2024 – Umbilical Cord MSCs in Moderate-to-Severe Crohn’s
Title: Therapeutic potential of human umbilical cord-derived mesenchymal stem cells in Crohn’s disease
Date: April 25, 2024
Link: News-Medical Summary / Research Report
Summary:
In a Chinese pilot study, patients with active Crohn’s unresponsive to standard therapy received local stem-cell injections during colonoscopy plus IV infusion. By 12 weeks, ~50% showed visible ulcer healing and some achieved complete mucosal repair. At 24 weeks, many reached clinical remission with no major side effects, suggesting MSC therapy may restore gut integrity safely.
Could Regenerative Medicine Be Right for You?
You may want to consider regenerative therapy if you:
- Experience frequent or severe flare-ups despite medication
- Struggle with side effects from steroids or biologics
- Want to explore non-surgical, restorative approaches
- Seek a therapy that focuses on repairing intestinal tissue, not just controlling symptoms
At Stemedix, our team carefully reviews your medical history and current therapies to determine if MSC-based regenerative treatment may complement your ongoing Crohn’s care.
Medical Disclaimer
This content is for educational purposes only and does not replace medical advice.
Stem-cell and regenerative therapies for Crohn’s disease are investigational and not FDA-approved.
Results vary; consult a licensed medical professional before undergoing treatment.
References
- Clinical, immunological, and radiographic improvements after Stem Cell Educator therapy. Int J Mol Sci, 2025.
- MSC therapy for perianal fistulizing Crohn’s: meta-analysis. Stem Cell Res Ther, 2025.
- Umbrella review: safety and efficacy of stem cell therapy for Crohn’s. PubMed Central, 2024.
- Therapeutic potential of UC-MSCs in Crohn’s disease. News-Medical Report, 2024.

by Shoot To Thrill Media | Oct 21, 2025 | Wrist & Hand Pain
Medical Review: Dr. Gerald Mastaw, MD – Board-Certified Physician
Last Updated: October 2025
What Causes Wrist & Hand Pain?
Your wrists and hands contain a complex network of joints, tendons, ligaments, and nerves that allow for precise movement and strength. Because they’re used constantly, typing, lifting, gripping, they’re also prone to strain, inflammation, and degeneration over time.
Common Causes
- Carpal tunnel syndrome (nerve compression in the wrist)
- Tendinitis or tenosynovitis (inflammation of tendons)
- Arthritis (wear of cartilage in small joints)
- Ligament or tendon tears
- Overuse or repetitive stress injuries
- Post-traumatic pain or stiffness after injury or surgery
Typical Symptoms
- Pain or stiffness in the wrist, thumb, or fingers
- Numbness or tingling (especially at night or with typing)
- Swelling or tenderness
- Weak grip or difficulty opening jars or holding objects
- Clicking, popping, or grinding with movement
These symptoms can worsen with daily use, making even simple tasks uncomfortable.
Traditional Treatment Options
Most people start with conservative care to reduce inflammation and restore motion.
Common approaches include:
- Rest or wrist splints
- Ice and anti-inflammatory medications
- Cortisone injections for short-term relief
- Physical or occupational therapy
- Ergonomic adjustments for repetitive motion
When these methods fail, some patients are advised to consider surgery for severe arthritis, ligament tears, or carpal tunnel decompression.
However, surgery can involve downtime, stiffness, and incomplete recovery, especially in older or highly active individuals.
Regenerative Medicine for Wrist & Hand Healing
Regenerative medicine offers a biologically driven approach to healing. Instead of masking symptoms, treatments like platelet-rich plasma (PRP) and umbilical cord tissue–derived mesenchymal stem cells (UCT-MSCs) may help repair and regenerate damaged tissues at a cellular level.
How It Works
- PRP Therapy: Uses concentrated growth factors from your own blood to stimulate healing in tendons, ligaments, and joint tissues.
- UCT-MSC Therapy: Delivers stem-cell-derived signaling molecules that reduce inflammation, modulate immune activity, and encourage tissue regeneration.
- These biologics can be injected precisely into the wrist or affected joints under ultrasound guidance.
Potential Benefits
- Reduced pain and swelling
- Improved grip strength and mobility
- Better tissue repair and flexibility
- Reduced reliance on steroids or pain medications
- Potential to delay or avoid surgery
Note: PRP and MSC-based therapies are not FDA-approved for wrist or hand conditions. They are considered investigational, though increasing clinical evidence supports their safety and therapeutic potential.
Recent Clinical Studies on Regenerative Treatments for Wrist & Hand Conditions
2025 – MSCs for Hand Osteoarthritis
Title: Umbilical cord-derived mesenchymal stem cell injections improve hand function and reduce pain in osteoarthritis: a phase I/II clinical trial
Date: January 2025
Link: PubMed Central
Summary:
In this early-phase human study, patients with painful hand and thumb osteoarthritis received a single injection of umbilical cord–derived MSCs. Over 12 months, they experienced significant improvements in hand pain (VAS) and function (DASH scores) with no serious adverse effects. MRI scans showed reduced inflammation in joint tissues. The authors concluded that UCT-MSCs are a safe and promising therapy for small-joint osteoarthritis of the hands.
2024 – PRP vs Corticosteroids for Wrist Tendinitis
Title: Efficacy of platelet-rich plasma versus corticosteroid injection in chronic wrist flexor and extensor tendinopathy: a randomized controlled trial
Date: October 2024
Link: Journal of Orthopaedic Surgery and Research
Summary:
This RCT compared PRP and steroid injections in 80 patients with chronic wrist tendinitis. Both groups improved, but PRP patients had greater pain relief and functional recovery at 6 months. Ultrasound follow-up showed better tendon thickness and vascularity in the PRP group, indicating true tissue repair rather than just symptom relief.
2023 – MSC Exosomes for Carpal Tunnel Syndrome
Title: Clinical evaluation of umbilical cord mesenchymal stem cell–derived exosomes for mild-to-moderate carpal tunnel syndrome
Date: July 2023
Link: Frontiers in Neurology
Summary:
In this first-of-its-kind pilot trial, 25 patients with carpal tunnel syndrome received a single ultrasound-guided injection of MSC-derived exosomes. By 3 months, participants reported significant pain reduction and improved nerve conduction velocity, with no safety issues. Researchers concluded that exosome therapy was safe and may enhance nerve recovery in carpal tunnel syndrome.
2022 – PRP for Thumb (CMC) Osteoarthritis
Title: Platelet-rich plasma injection for first carpometacarpal (CMC) joint osteoarthritis: a randomized controlled trial
Date: February 2022
Link: Journal of Hand Surgery (European Volume)
Summary:
Fifty patients with thumb base arthritis received either PRP or hyaluronic acid injections. Both groups improved, but PRP patients reported stronger grip, less pain, and better hand function at 6 months. The authors noted PRP is a safe, non-surgical alternative that provides sustained pain relief for thumb arthritis.
2021 – PRP for Chronic Wrist Ligament Injuries
Title: Autologous platelet-rich plasma in chronic wrist ligament injury: a prospective clinical study
Date: August 2021
Link: BMC Musculoskeletal Disorders
Summary:
Patients with chronic scapholunate ligament injuries (a common cause of wrist instability) received PRP injections under fluoroscopic guidance. Over 12 months, they showed improved grip strength, stability, and reduced pain, without surgical intervention. Imaging demonstrated ligament thickening and improved continuity in several cases.
Is Regenerative Care Right for You?
You may be a good candidate if you:
- Have chronic wrist or hand pain that hasn’t improved with standard care
- Suffer from carpal tunnel, arthritis, or tendon inflammation
- Want to avoid surgery or long recovery times
- Prefer a natural, cell-based therapy focused on healing
At Stemedix, our team evaluates your history, imaging, and goals to determine whether PRP, stem-cell-derived biologics, or a combination approach may best support your recovery.
Medical Disclaimer
This information is for educational purposes only and is not medical advice.
PRP and stem cell therapies for wrist and hand pain are investigational and not FDA-approved.
Individual results vary; always consult a licensed healthcare provider.
References
- Umbilical cord-derived mesenchymal stem cells for hand osteoarthritis. PubMed Central, 2025.
- PRP vs corticosteroid injection in wrist tendinopathy: RCT. Journal of Orthopaedic Surgery and Research, 2024.
- MSC-derived exosomes for carpal tunnel syndrome. Frontiers in Neurology, 2023.
- PRP for first carpometacarpal (thumb) arthritis: RCT. Journal of Hand Surgery (European Volume), 2022.
- PRP in chronic wrist ligament injury: prospective study. BMC Musculoskeletal Disorders, 2021.

by Shoot To Thrill Media | Oct 21, 2025 | Post-Stroke Syndrome
Medical Review: Dr. Gerald Mastaw, MD – Board-Certified Physician
Last Updated: October 2025
What Is Post-Stroke Syndrome?
Post-Stroke Syndrome refers to the lasting physical, cognitive, and emotional effects that can occur after a stroke.
While some people recover rapidly, others may experience ongoing challenges that persist for months or even years.
Common Symptoms Include:
- Muscle weakness or stiffness
- Difficulty speaking or understanding words
- Memory or concentration problems
- Persistent fatigue or low energy
- Mood changes such as depression or anxiety
- Chronic pain, numbness, or tingling sensations
These symptoms vary in severity from person to person, but they can significantly impact independence, mobility, and quality of life.
Conventional Treatment Approaches
Traditional stroke rehabilitation focuses on restoring function and preventing complications, but progress often slows after the first few months.
Treatment typically combines physical, occupational, and speech therapy along with symptom management.
Common Treatments Include:
- Physical therapy – Improves strength, coordination, and balance
- Occupational therapy – Helps patients relearn everyday skills
- Speech therapy – Assists with communication and swallowing difficulties
- Medications – Manage depression, spasticity, pain, or sleep disturbances
- Psychological support – Addresses mood and cognitive changes
While these therapies remain essential, some patients reach a plateau in recovery. This is where regenerative medicine may provide new potential for continued improvement.
Regenerative Medicine: Supporting Brain Repair
Regenerative medicine aims to activate the body’s own repair mechanisms to restore tissue health and function.
One of the most promising areas involves umbilical cord tissue-derived mesenchymal stem cells (UCT-MSCs), which are being studied for their ability to support neural recovery after stroke.
How UCT-MSC Therapy May Help
Research suggests that UCT-MSCs may:
- Protect and repair damaged brain tissue
- Reduce inflammation that worsens neurological injury
- Enhance communication between surviving nerve cells
- Support motor and cognitive recovery
- Improve overall energy, focus, and coordination
These cells release natural growth factors that can help create a more favorable environment for healing, even in chronic post-stroke phases where traditional therapies have plateaued.
Important Note:
Stem cell therapy for stroke recovery is investigational and not FDA-approved. However, early research suggests that biologic and cellular approaches may help improve quality of life and function for some patients.
Recent Clinical Studies on Regenerative Medicine for Stroke Recovery
2025 – Cord Blood Infusion for Acute Ischemic Stroke
Title: Allogeneic Human Umbilical Cord Blood for Acute Ischemic Stroke: Phase I Clinical Trial
Journal: PubMed – Full Text
Summary:
In this Phase I study, six adults received umbilical cord blood infusions within 9 days of stroke onset.
The treatment was well-tolerated with no serious adverse events.
Over the following year, patients showed steady improvement in movement, speech, and daily function, reflected by better neurological and independence scores.
Researchers concluded that cord blood infusions are safe and may enhance recovery after ischemic stroke.
2023 – UC-MSC Therapy in Chronic Stroke Patients
Title: Outcomes of Mesenchymal Stem Cell Transplantation in Five Stroke Patients
Journal: Frontiers in Medicine – Full Text
Summary:
Five adults with long-term post-stroke disability received umbilical cord MSC infusions.
All showed notable recovery in strength, coordination, and mobility—even years after their strokes.
One participant regained arm and hand movement after years of paralysis.
No serious side effects were reported.
The study highlighted that regenerative therapy may help restore function beyond the typical rehabilitation window.
2022 – Direct Brain Injection of Placenta-Derived MSC Exosomes
Title: Safety of Intraparenchymal Injection of Allogeneic Placenta MSC-Derived Exosomes in Severe Stroke Patients: Pilot Randomized Trial
Journal: Frontiers in Neurology – Full Text
Summary:
In this small pilot trial, five patients with severe brain swelling received exosome injections (healing nanoparticles from placenta MSCs) during surgery.
No bleeding, swelling, or immune reactions occurred.
Some patients showed early neurological improvement.
The findings confirmed procedure safety and laid the groundwork for larger studies on cell-free regenerative stroke therapies.
2021 – Cord Blood Monocyte Infusion Restores Motor Function
Title: Complete Restoration of Motor Function in Acute Cerebral Stroke Treated with Allogeneic Cord Blood Monocytes: Phase I Trial
Journal: PubMed – Full Text
Summary:
A 51-year-old man who was nearly paralyzed after a major stroke received cord blood monocyte infusion.
Over one year, he regained full independence and movement, with imaging showing shrinkage of the brain injury area.
No adverse reactions were seen, suggesting cord-derived cells can enhance brain repair safely and effectively.
2021 – UC-MSC Therapy After Ischemic Stroke
Title: Treatment of Acute Ischemic Stroke by Minimally Manipulated Umbilical Cord-Derived MSC Transplantation: Case Report
Journal: PubMed – Full Text
Summary:
A 45-year-old patient received three UC-MSC infusions following stroke.
Over the next year, he experienced steady recovery in speech, strength, and independence, with brain imaging confirming reduced injury size.
The therapy was safe and well tolerated, and his neurological condition improved to near-normal levels.
This case demonstrates the potential of UCT-MSC therapy to support functional recovery after stroke.
Is Regenerative Therapy Right for You?
Stem cell and regenerative therapies may be worth exploring if you:
- Have persistent weakness, speech issues, or fatigue after stroke
- Have plateaued in traditional rehabilitation
- Are seeking a non-surgical, biologic option to support brain recovery
- Want to learn about evidence-informed therapies that promote neurorepair
At Stemedix, we provide comprehensive evaluations to determine whether regenerative care could complement your recovery plan. Our focus is on helping patients regain independence and enhance quality of life through advanced, science-based approaches.
Medical Disclaimer
This page is for educational purposes only and does not replace professional medical advice.
Stem cell and exosome therapies for post-stroke recovery are not FDA-approved, and results vary by individual.
Always consult your healthcare provider before pursuing any investigational treatment.
References
- Lee H. et al. Allogeneic Human Umbilical Cord Blood for Acute Ischemic Stroke. PubMed., 2025. Full Text
- Park M. et al. Outcomes of Mesenchymal Stem Cell Transplantation in Chronic Stroke. Front Med., 2023. Full Text
- Farid H. et al. Placenta MSC-Derived Exosomes for Severe Stroke. Front Neurol., 2022. Full Text
- Ouyang Q. et al. Cord Blood Monocyte Infusion in Acute Cerebral Stroke. PubMed., 2021. Full Text
- Zhang Y. et al. UC-MSC Therapy for Ischemic Stroke. PubMed., 2021. Full Text





 St. Petersburg, Florida
St. Petersburg, Florida
