
by admin | May 5, 2023 | Health Awareness, Multiple Sclerosis
The fatigue, mobility and balance issues, and muscle spasms that commonly occur with multiple sclerosis (MS) can make exercising seem impossible. However, staying active is critical to managing MS symptoms, avoiding injuries, and maintaining independence.
Choosing the Right Exercises for You
Even patients with very advanced cases of MS can find a form of exercise that reduces fatigue and inflammation and improves strength and balance.
The most beneficial exercises for those with MS center on four primary focuses: aerobics, strength, flexibility, and balance.
Aerobic Exercises for MS
Aerobic exercise can improve cardiovascular health, fatigue, and mood. Low-impact aerobic exercises include walking, biking, swimming, and using an elliptical machine.
In addition, patients with muscle spasticity in their legs may benefit from using a stationary bike where they can clip in, allowing them to keep their feet on the pedals without extra effort.
Strength Exercises for MS
Resistance training, bodyweight workouts, and progressive strength training using dumbbells or barbells can increase stamina, build muscles, and improve bone density.
Depending on symptoms and mobility, bodyweight workouts, such as pushups or squats, can strengthen muscles without worrying about dropping weights or holding onto resistance bands.
Progressive strength exercises allow you to gradually increase your resistance or weights to build muscle mass and improve physical function. For example, bicep curls, deadlifts, shoulder presses, and rows build upper body strength and allow for progressive muscle building.
Flexibility Exercises for MS
Daily stretching, either from a yoga class or just a few minutes of working on touching toes and moving the spine, can increase the range of motion and decrease muscle spasticity.
Focus on spastic muscles, and aim to hold your stretches for 30 seconds to a minute to fully reap the muscle-lengthening benefits.
Balance Exercises for MS
Balance training focuses on posture, shifting body weight, and creating stability, so patients with MS can reduce their risk of falls and maintain independence. While yoga and Pilates are both beneficial for balance training, even standing on one leg while brushing your teeth or doing the dishes can significantly improve overall balance.
If balancing exercises are challenging, try them next to a wall or while holding onto a chair to maintain stability as your balance improves.
By including daily exercises that allow for long-term benefits, MS patients can regain some control over their condition and symptoms.

by Stemedix | Apr 17, 2023 | Multiple Sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system (CNS). It occurs when the immune system mistakenly attacks and damages the protective covering of nerve fibers, called myelin, in the brain and spinal cord. A very commonly asked question is ” How does Multiple Sclerosis affect the body? “. Keep reading to learn more!
The myelin sheath is a protective covering that surrounds nerve fibers in the central nervous system (CNS). It is made up of a fatty substance called myelin, which is produced by specialized cells called oligodendrocytes in the CNS. The myelin sheath acts as an insulator and helps to speed up the transmission of electrical impulses along the nerve fibers.
The myelin sheath is important for the proper functioning of the nervous system. When it is damaged, nerve impulses can slow down or disrupt communication between the brain and other parts of the body, leading to a wide range of neurological symptoms.
In MS, when the myelin sheath is damaged, it can cause a wide range of symptoms. The severity and frequency of symptoms can vary widely between individuals with MS. Some of the most common symptoms of MS include:
- Muscle weakness and stiffness: MS can cause weakness and stiffness in the muscles, which can affect mobility and balance.
- Numbness and tingling: MS can cause numbness and tingling in the limbs, face, and other parts of the body.
- Vision problems: MS can affect the optic nerve, which can cause blurred or double vision, loss of vision, or pain behind the eyes.
- Fatigue: MS can cause extreme tiredness and lack of energy, even after minimal physical or mental activity.
- Cognitive problems: MS can affect cognitive functions such as memory, concentration, and problem-solving.
- Bladder and bowel problems: MS can cause problems with bladder and bowel function, such as incontinence or constipation.
- Emotional changes: MS can cause mood swings, depression, and anxiety.
The symptoms of MS can be unpredictable and can vary in severity over time. Treatment can help manage symptoms and slow the progression of the disease, but there is currently no cure for MS.
What Treatments or Therapies Help Multiple Sclerosis?
The exact cause of MS is not known, but it is thought to be a combination of genetic and environmental factors. There is currently no cure for MS, but treatments are available that can help manage symptoms, slow the progression of the disease, and improve quality of life.
The choice of treatment depends on the type and severity of MS, as well as the individual’s symptoms and overall health. Some options include:
Disease-modifying therapies (DMTs): These are medications that are used to slow down the progression of MS, reduce the frequency and severity of relapses, and help preserve cognitive function. There are several different types of DMTs available, including injectable medications, oral medications, and infusion therapies. As a medication, DMTs may cause side effects, which can vary depending on the medication. Common side effects include flu-like symptoms, injection site reactions, and gastrointestinal problems.
Overall, the decision to use DMTs should be made in consultation with a healthcare provider, taking into account the individual’s specific needs, goals, and potential risks and benefits.
Symptom management: MS can cause a wide range of symptoms, and there are several medications and therapies that can help manage these symptoms. For example, medications can be used to reduce muscle spasms, pain, and bladder and bowel problems. Physical therapy, occupational therapy, and speech therapy can also be helpful in managing symptoms.
Lifestyle changes: Certain lifestyle changes, such as regular exercise, a healthy diet, and stress management techniques, can help improve overall health and reduce the risk of MS relapses.
Exercise: Exercise can be an important part of managing multiple sclerosis (MS), as it can help improve strength, balance, flexibility, and overall quality of life. However, the best exercise for MS can vary depending on the individual’s symptoms and overall health. Some examples are aerobic exercise, strength training, yoga or tai chi, and water based exercise.
Diet: There is no one-size-fits-all diet for multiple sclerosis (MS), and the best diet for MS may vary depending on the individual’s symptoms and overall health. However, research suggests that a healthy, balanced diet can help improve overall health and well-being for people with MS. Here are some general principles of a healthy diet that may be beneficial for people with MS:
- Focus on whole foods: A diet rich in whole, nutrient-dense foods can help provide the vitamins, minerals, and antioxidants needed for optimal health. This includes fresh fruits and vegetables, whole grains, lean proteins, and healthy fats.
- Avoid processed foods: Processed foods, such as packaged snacks and sugary drinks, are often high in sugar, salt, and unhealthy fats. These foods can contribute to inflammation and may worsen MS symptoms.
- Consider an anti-inflammatory diet: Inflammation is thought to play a role in the development and progression of MS. Eating an anti-inflammatory diet, which includes foods such as fatty fish, nuts, seeds, and olive oil, may help reduce inflammation in the body.
- Supplement as needed: Some people with MS may have specific nutrient deficiencies, such as vitamin D or vitamin B12. In these cases, supplementation may be necessary to meet the body’s needs.
- Stay hydrated: Drinking plenty of water can help keep the body hydrated and may help reduce MS-related symptoms such as fatigue and constipation.
Stress Management: Stress is a common trigger for MS symptoms, so it is important for people with MS to learn stress management techniques to help them manage their condition. Here are some stress management techniques that may be helpful for people with MS. Some include meditation, breathing exercises, yoga, cognitive-behavioral therapy (CBT), and regular exercise.
Rehabilitation: Rehabilitation programs can help individuals with MS maintain or improve their physical and cognitive abilities. They can also help to manage symptoms, promote independence, and improve mental health. These programs may include physical therapy, occupational therapy, and speech therapy.
Alternative therapies: Some people with MS find that alternative therapies can be helpful in managing their symptoms and improving their quality of life. Here are some alternative therapies that some people with MS may find helpful:
- Acupuncture: Acupuncture involves the insertion of thin needles into specific points on the body. Some studies suggest that acupuncture may help relieve pain, fatigue, and other MS-related symptoms.
- Massage therapy: Massage therapy involves the manipulation of soft tissues to promote relaxation and relieve muscle tension. Some people with MS may find massage therapy helpful in reducing muscle spasms and improving overall relaxation.
- Mind-body therapies: Mind-body therapies, such as yoga, tai chi, and meditation, can help improve flexibility, balance, and relaxation. These practices may also help reduce stress and improve mood.
- Herbal remedies: Some herbal remedies, such as turmeric, ginkgo biloba, and omega-3 fatty acids, may have anti-inflammatory properties that could potentially help reduce inflammation in the body.
- It is important for individuals with MS to talk to their healthcare provider before starting any new alternative therapy, as some therapies may not be appropriate for certain symptoms or health conditions.
Regenerative Medicine for Multiple Sclerosis
Regenerative medicine, also known as stem cell therapy, is a rapidly evolving area of research and has shown promise in the treatment of multiple sclerosis (MS). Stem cells are unspecialized cells that have the potential to develop into many different types of cells to help repair damaged tissues or cells and reduce inflammation.
Mesenchymal stem cell (MSC) therapy is a type of regenerative medicine that uses stem cells derived from various tissues, including bone marrow, adipose tissue, and umbilical cord tissue, to treat a variety of conditions, including multiple sclerosis (MS).
Several clinical trials have investigated the use of MSC therapy in MS, and some have shown promising results. MSCs have anti-inflammatory and immunomodulatory properties, which may be beneficial in the treatment of MS. MSCs can also promote the regeneration of damaged tissue, which may help improve symptoms.
One small clinical trial published in 2018 showed that treatment with MSCs improved clinical outcomes and reduced inflammation in individuals with MS. Another study published in 2019 showed that MSCs derived from umbilical cord tissue reduced inflammation and improved motor function. As with any medical treatment, the decision to undergo MSC therapy for MS should be an informed decision and with a provider that has experience and has a positive reputation. Would you like to speak with a professional to help answer the question ” How does Multiple Sclerosis affect the body? “. Contact a care coordinator today at Stemedix to learn more!

by Stemedix | Apr 3, 2023 | Multiple Sclerosis, Regenerative Medicine, Stem Cell Therapy
Multiple sclerosis (MS) is a chronic and progressive neurological disease that affects the central nervous system (CNS), which includes the brain and spinal cord. MS occurs when the immune system mistakenly attacks the myelin, a fatty material that surrounds and protects nerve fibers, causing inflammation and damage to the myelin and the nerve fibers themselves. Many people often wonder ” Is Multiple Sclerosis hereditary? Keep Reading to find out!
The symptoms of MS can vary widely depending on the location and extent of the damage to the CNS. Common symptoms include fatigue, weakness, balance problems, difficulty walking, numbness or tingling sensations, blurred or double vision, muscle stiffness and spasms, bladder and bowel problems, and cognitive impairment.
How is Multiple Sclerosis Diagnosed?
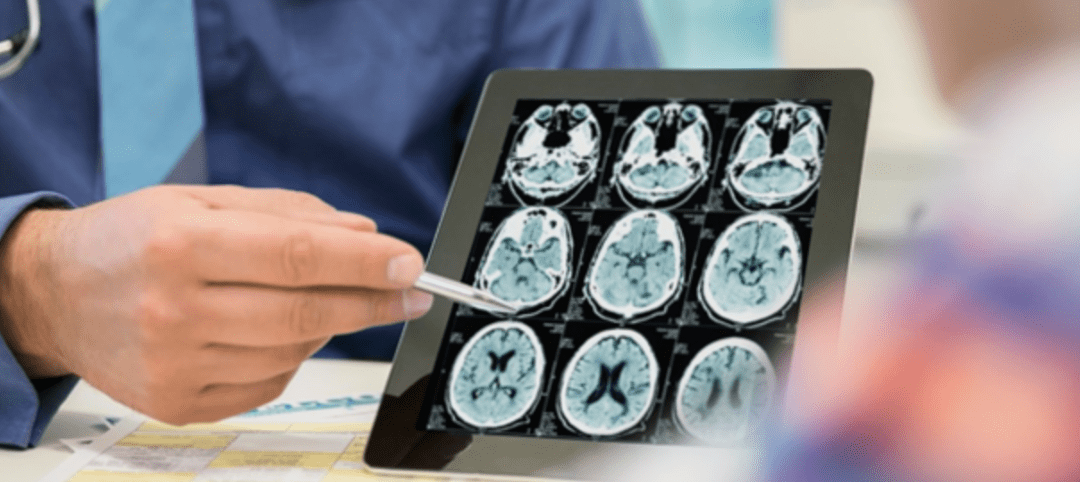
In general, the diagnosis of MS is made based on a combination of clinical symptoms, physical examination, and diagnostic tests, such as magnetic resonance imaging (MRI) and cerebrospinal fluid analysis. While these tests cannot definitively determine the cause of MS, they can help to identify characteristic patterns of damage in the CNS that are consistent with the disease.
The identifying characteristic patterns of damage in the central nervous system (CNS) for multiple sclerosis (MS) can be seen on magnetic resonance imaging (MRI) scans and include the following:
Multiple lesions: MS typically causes multiple areas of damage, or lesions, in the CNS. These lesions can appear in various regions of the brain and spinal cord and are often visible on MRI scans as bright or dark spots.
White matter damage: MS primarily affects the myelin sheath, which is a fatty substance that surrounds nerve fibers in the white matter of the brain and spinal cord. The damage to the myelin results in the formation of lesions that can be seen on MRI scans.
Inflammation: MS is caused by an abnormal immune response that results in inflammation in the CNS. This inflammation can be seen on MRI scans as areas of increased brightness, indicating increased blood flow and immune cell activity.
Symmetry: MS lesions tend to occur in a symmetric pattern, meaning they appear in similar locations on both sides of the brain or spinal cord.
Time course: MS lesions can appear and disappear over time, and new lesions may develop while old lesions may heal. This pattern of damage over time is a key diagnostic feature of MS.
Overall, the combination of multiple lesions, white matter damage, inflammation, symmetric involvement, and a relapsing and remitting time course seen on MRI scans can help to distinguish MS from other neurological conditions that can cause similar symptoms.
Is Multiple Sclerosis Caused by Heredity or Environmental?
Multiple sclerosis (MS) has a complex etiology and while the cause of MS is not fully understood, research suggests that a combination of genetic and environmental factors may contribute to its development. Currently, there are no definitive tests to determine whether the condition is caused by genetic or environmental factors alone.
People with a family history of MS, certain infections, and vitamin D deficiency are thought to be at increased risk for the disease. Having a close relative with MS, such as a parent or sibling, does increase a person’s risk of developing the disease. However, the risk is still relatively low, with most people with a family history of MS not developing the disease themselves.
While there has been no single gene identified as the cause of the disease responsible for MS and appears to be complex and multifactorial. Genetic testing can be used to identify certain genes that may increase the risk of developing MS but it is not directly inherited in a simple Mendelian fashion, where a single gene is responsible for the disease and follows a predictable pattern of inheritance. Instead, it is believed that multiple genes, each contributing a small effect, interact with environmental factors to increase the risk of developing MS.
Environmental factors, such as exposure to certain infections, smoking, and low vitamin D levels, have also been linked to an increased risk of developing MS. However, it can be challenging to determine the precise environmental factors that contribute to the disease, as many factors may be involved, and their effects may be difficult to measure.
Overall, while genetics can play a role in the development of MS, it is a complex disease with multiple factors contributing to its onset, and more research is needed to fully understand its genetic basis.
Treatments for Multiple Sclerosis
MS is a lifelong disease with no known cure, but there are treatments available to help manage the symptoms and slow the progression of the disease. Traditional medicine may include medications to reduce inflammation and modulate the immune system, physical therapy to improve mobility and balance, occupational therapy to help with activities of daily living. But some are also exploring regenerative medicine.
What is Regenerative Medicine for MS?
Regenerative medicine, also known as stem cell therapy, is an interdisciplinary field that seeks to replace or regenerate damaged or diseased tissues. This new alternative medicine has the potential to help slow down progression and manage symptoms.
Stem cells are undifferentiated cells that can develop into different types of cells in the body. The most common stem cell used in therapy today is the mesenchymal stem cell which can be derived from adipose (fat), umbilical cord, or bone marrow tissues.
In MS, stem cell therapy involves using mesenchymal stem cells (MSCs) to regenerate damaged myelin and nerve fibers in the CNS. These MSCs can modulate the immune response and reduce inflammation, which can help to prevent further damage to the myelin sheath that surrounds and protects neurons. Studies have shown that stem cell therapy can improve neurological function and reduce disease activity in some patients with MS.
While regenerative medicine approaches for MS are still in the early stages of development, they hold great promise for the future treatment of this complex disease. To learn more about Regenerative Medicine and the different options for Multiple Sclerosis ( MS ) contact a care coordinator today at Stemedix!

by Stemedix | Jan 23, 2023 | Multiple Sclerosis
One of the challenges of diagnosing and managing early signs of multiple sclerosis (MS) is the condition’s unpredictable nature. Those with MS may experience chronic, severe symptoms or be largely symptom-free. So what are the first signs of having Multiple Sclerosis?
The early signs of MS can be varied or overlap with symptoms of other illnesses. However, a proper diagnosis is critical, as early MS treatments can delay the disease’s progression. Most adults with MS in the U.S. receive their diagnoses between the ages of 20 and 50.
What Is Multiple Sclerosis?
As an autoimmune disease, MS occurs when the body’s immune system mistakenly attacks its healthy cells. Patients with MS experience attacks on the nerves’ protective coating, called myelin or the myelin sheath. Attacks on myelin damage the nerves, causing problems throughout the central nervous system.
As the attacks seem to target nerves at random, symptoms vary widely.
What Are the Early Signs of MS?
Some of the more common early signs of MS include:
- Tingling and numbness
- Weakness
- Balance problems or dizziness
- Vision problems
- Pain
- Muscle spasms or cramps
- Bladder issues
- Fatigue
- Cognitive problems
- Sexual dysfunction
In some patients with MS, the condition initially presents with neurological symptoms that have no other known cause and last at least 24 hours. These symptoms are known as a clinically isolated syndrome (CIS).
CIS doesn’t always lead to MS, but since CIS results from demyelination or damage to the myelin that occurs with MS, it often serves as an early sign of the disease.
For example, a CIS episode could include optic neuritis resulting from damage to the myelin of the optic nerve that causes eye pain and vision issues. Another condition associated with CIS is transverse myelitis, which can cause muscle weakness or numbness.
Diagnosing MS
There isn’t a standard test to diagnose MS. Instead, most physicians start by ruling out other medical conditions with similar symptoms. For example, Lyme disease, diabetes, nerve damage, and lupus are sometimes mistaken for MS, and vice versa.
After a thorough exam and medical history, your doctor may request testing to determine whether you have MS. Blood tests, a spinal tap, and an MRI can help your physician rule out other conditions or identify telltale signs of MS, such as lesions on your brain or spinal cord.
Most patients have relapsing-remitting MS, which typically allows for a more straightforward diagnosis. However, those with unusual symptoms or an uncommon disease progression may require further testing.
Managing Symptoms of MS
Aggressively managing early signs of MS as soon as possible has the potential to slow the disease’s progression. That’s why identifying early symptoms is critical to maintaining your health.
Many patients are discovering the promising therapy of regenerative medicine. This therapy utilizes stem cells to offer a new alternative option to potentially help repair damaged tissues, reduce inflammation, and slow down the progression of MS.
Those looking for options when traditional medicine has not provided ideal results or has not been successful may want to explore this therapy to see if it is for them. To learn more about the options you have after you see the first signs of having Multiple Sclerosis, contact a care coordinator today at Stemedix!

by admin | Jul 27, 2022 | Multiple Sclerosis, Health Awareness
Multiple sclerosis (MS) is an autoimmune disease in which the immune system attacks the brain, spinal cord, and the rest of the central nervous system. The National Multiple Sclerosis Society states there are, on average, one million people in the United States living with this disease.
One treatment that has the potential of offering an improvement in the symptoms of MS is biotin. Learn what biotin is and what it can offer.
What Is Biotin?
Biotin is also known as vitamin B7. It is one of the B-complex vitamins essential for nerve cell metabolism and is present in many foods, including:
- Egg yolks
- Chard
- Brewer’s yeast
- Nuts
- Livers
- Soybeans and other legumes
- Whole grains
- Bananas
- Mushrooms
- Cauliflowers
Biotin is a part of enzymes that break down fats, carbohydrates, amino acids, and other substances. It also has links to healthy nails, hair, and skin.
How Biotin Can Help Combat MS
Biotin can work to combat MS by stimulating enzymes that help produce more myelin. Myelin is a layer that wraps around your nerves and increases the rate at which electrical impulses travel, and when the immune system targets and damages myelin, multiple sclerosis develops.
Having healthy levels of myelin makes it possible for nerve cells to communicate better, which can relieve symptoms of MS and might even slow the disease’s progression. As such, a supplement such as biotin that encourages myelin production can be very helpful in managing MS.
In some studies, people with MS saw an improvement in their vision upon taking biotin. Another study showed that those taking high doses of biotin felt a reduction in pain levels as well as a boost in energy and a reduction in paralysis.
Learn More About Biotin
Biotin has the potential to offer relief from symptoms of multiple sclerosis. Whether it is the right choice for you will depend on many factors, including your doctor’s recommendations.
If you have MS and are interested in exploring biotin, contact us to learn how you can order today.

by admin | May 6, 2022 | Stem Cell Therapy, Mesenchymal Stem Cells, Multiple Sclerosis, Stem Cell Research
Multiple sclerosis (MS) is a chronic inflammatory disease that attacks myelin, the protective sheath that covers nerves and causes progressive and serious communication issues between the brain, central nervous system, and the rest of the body[1].
Currently, it’s estimated that over 2.3 million people worldwide, and over one million people in the US have a diagnosis of MS[2].
While there have been significant improvements in treatments designed to stabilize, delay, and even improve symptoms of MS, new and more effective treatments are needed to improve the long-term outcome associated with the condition.
One area currently being investigated as a potential therapeutic option for treating MS is the use of regenerative medicine, also known as stem cell therapy, and specifically treatment using mesenchymal stem cells (MSCs).
In this review of evidence from preclinical and clinical studies, Gugliandolo et al. examine studies involving the use of MSCs or their derivatives in vivo models of MS and patients affected by MS. The authors also examine and discuss the feasibility of autologous MSCs therapy for MS patients.
Specifically, and when assessed in terms of effectiveness when treating MS, the therapeutic potential of MSCs was associated with their differentiation capacity and paracrine effects, their ability to differentiate toward oligodendrocytes and express oligodendrocyte progenitor cell (OPC) markers, and their capacity for homing (moving towards the damaged area following chemical gradients).
As part of this review, the authors also examined the effectiveness of various sources of MSC in MS models, these sources included bone marrow MSCs (BM-MSCs), adipose tissue-derived MSCs (AD-MSCs), periodontal ligament stem cells (PDLSCs), skin-derived MSCs (S-MSCs), Wharton’s jelly-derived MSCs (WJ-MSCs), human umbilical cord MSCs (UCMSC), human amnion mesenchymal cells (AMCs), placental derived MSCs (PMSCs), and decidua derived MSCs (DMSCs). According to the research reviewed by Gugliandolo et al., all MSCs, regardless of where they were harvested from, demonstrated beneficial effects in the therapeutic treatment of MS.
Specifically, the results demonstrated that MSCs were able to produce some form of protective effects in reducing inflammatory cell infiltration, disease score, demyelination, and blood-brain barrier disruption.
A review of 29 phase 1 or 2 clinical trials registered on clinicaltrials.gov demonstrated that MSCs, regardless of the type and method of administration, demonstrated to be safe and absent of severe adverse effects with the majority demonstrating measurable improvements when used in MS patients.
While clinical trials demonstrated the safety of administration of MSC in MS patients, the authors were particularly interested in learning if autologous MSC transplantation presented some advantages over heterologous administration.
The authors of this review found that samples obtained from healthy controls and MS patients showed similar features, indicating the possibility of autologous stem cell therapy in MS patients. However, other studies found that MSCs obtained from MS patients exhibited a different transcriptional pattern and fewer immunosuppressive functions compared to healthy donor MSCs.
Gugliandolo et al. point out that limits to these experimental studies include the use of animals of a single gender, given that sex-dependent differences exist and the use of different MS models, different number of transplanted cells, different MSCs sources, and routes of administration. These limitations make it difficult to define the optimal treatment in terms of cell type, dose, and administration conditions.
The authors conclude that clinical trials demonstrate the safety and feasibility of MSCs treatment, and also some improvements, but more data on larger cohorts are required to establish their efficacy. Considering the controversial results pertaining to the features of MSCs derived from MS patients, the authors also call for additional research in order to conclusively determine the safety and efficacy of autologous MSCs therapy in MS patients.
Source: “Mesenchymal Stem Cells in Multiple Sclerosis – NCBI.” 17 Nov. 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7698327/.
[1] “Multiple sclerosis – Symptoms and causes – Mayo Clinic.” 7 Jan. 2022, https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/symptoms-causes/syc-20350269.
[2] “Understanding MS | National Multiple Sclerosis Society.” https://www.nationalmssociety.org/What-is-MS/MS-FAQ-s.





 St. Petersburg, Florida
St. Petersburg, Florida
