
by admin | Sep 3, 2018 | Multiple Sclerosis
Foot drop, also commonly referred to as drop foot, is a condition in which the muscles that lift the foot become weak or paralyzed. As a result, the foot may drag while walking. Foot drop isn’t a disease in itself; instead, it can develop as a result of preexisting medical conditions. Learn more about what causes the symptom and how it can be treated below.
What Are the Characteristics of Foot Drop?
Because foot drop causes the front toes to drag, it often leads individuals with the condition to overcorrect their gait to avoid tripping or discomfort. They may either swing the leg outward in an arc or lift the knee higher. This coping mechanism is what’s known as “steppage gait.”
Who Might Be Affected by Foot Drop?
Foot drop can be caused by certain neurological disorders, including amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), Multiple Sclerosis (MS), and muscular dystrophy. These and other neurological conditions lead to weakening and deterioration of the muscles, which can cause foot drop. Cerebral palsy and stroke may also bring on foot drop.
In addition to individuals with neurological conditions, foot drop can also occur in people who have sustained nerve damage, or neuropathy. Specifically, the peroneal nerve, which extends from the sciatic nerve and wraps from behind the knee to the shin, is compromised in drop foot. There are a number of possible causes of this form of nerve damage, including diabetes, sports injuries, hip or knee replacements or time spent in a cast, long durations spent cross-legged or squatting, and childbirth.
How is it Treated?
Physicians make treatment recommendations based on the severity of the condition, as well as its root cause. While it is not always fully curable, some treatments can make noticeable improvements in gait. Leg braces may be worn to provide ample support. Patients may also benefit from attending physical therapy to perform leg and foot strengthening exercises. Certain movements can also be completed at home, including gentle stretches and chair exercises.
Sometimes, functional electrical stimulation is also used to enhance nerve functionality, which can spur muscle contraction to lift the foot. Doctors may also recommend nerve surgery if it is deemed to be a feasible solution. For individuals who would not benefit from surgery, implementing lifestyle changes such as eliminating floor clutter and ensuring homes are well-lit may be helpful.

by admin | Jul 31, 2018 | Health Awareness
A healthy diet is important for feeling your best, but for individuals with an autoimmune disorder such as multiple sclerosis (MS), healthy eating plans become even more critical. This is the belief on which the Wahls diet was founded. Developed by Dr. Terry Wahls, the diet implements paleo-style eating to aid in symptom management. Here, we learn more about the eating plan that has helped MS patients and sufferers of other autoimmune disorders manage their conditions more effectively.
How is Diet Linked to Autoimmune Diseases?
Autoimmune disorders are suspected to be caused by low-grade inflammation, or the inflammation that takes place in our cells. Research suggests that a microbial imbalance of gut flora could also contribute to autoimmunity. Eating plans such as the Wahls protocol diet aim to reduce inflammation by eliminating certain food chemicals which could contribute to gut dysbiosis and inflammation in sensitive individuals.
What Does the Wahls Diet Entail?
Like the Paleolithic (Paleo) diet, the Wahls protocol emphasizes the consumption of meat and fish and vegetables. It also encourages fat intake from both animal and plant sources and allows for brightly-colored fruits, such as berries, to be enjoyed regularly.
In order to minimize potential inflammatory agents found in common food sources, the diet is fairly restrictive. For instance, dairy products and eggs are prohibited, along with nightshade vegetables such as eggplant, tomatoes, potatoes, and peppers. Legumes, all grains, and sugars (except for those occurring naturally in fruits) are also restricted.
In recognition of the varying degree of severity in autoimmune disorders, as well as patients’ diverse food preferences and needs, Dr. Wahls has established three tiers of the diet. For instance, Level 3 calls for the elimination of all white-fleshed fruits, while Level 1 requires only the avoidance of all foods containing gluten and dairy.
Does the Diet Really Work?
In patients with MS, following a paleo-style diet has been shown to improve fatigue. Yet, because subjects involved in clinical studies are also typically receiving additional forms of therapy, it is difficult to isolate dietary tactics alone as the primary agent for achieving results. Nonetheless, Dr. Wahls attributes the diet to her own reversal of symptoms. Before she embarked on a healthier eating plan, Dr. Wahls’ muscles had weakened to the point at which she needed a tilt-recline wheelchair. After transforming her diet, she was able to bike nearly 20 miles a day.
While research on the complex ways in which dietary choices impact immune functionality is still ongoing. For patients with autoimmune disorders like MS, talking to doctors about anti-inflammatory eating plans is certainly not a bad idea. Beyond aiding with symptom management, a healthier eating plan could support better wellness outcomes and reduce risks for other serious conditions, such as cardiovascular disease and type 2 diabetes.

by admin | Jul 27, 2018 | Health Awareness, Lupus, Multiple Sclerosis, Parkinson's Disease, Studies
Colostrum is the milk produced by the mammary glands during pregnancy prior to giving birth. It is rich in antibodies that help prevent the newborn from various conditions. Colostrum as compared to normal milk contains a high amount of nutrients and fat, making it highly beneficial.
The most important thing to know about colostrum is that it is not a medication. It is a naturally designed food that maintains the health and prevents conditions. Colostrum is effective for shutting down the onset of conditions and infections, which helps the body to repair itself and allows the individual to enjoy a healthy and radiant life.
Colostrum is the Key to Gut Health
Colostrum is the source of everything that is required to maintain a healthy gastrointestinal tract. It is known that most of our conditions take birth in the gut and proper absorption of nutrients is the key to great health. It is one of the primary function of colostrum to maintain a healthy gut, which is the basis of the overall healthy body.
When the beneficial bacteria present in our intestine is outnumbered by the harmful bacteria then our gut is said to be out of balance. This imbalance has many consequences, one of which is the leaky gut syndrome.
Leaky gut syndrome is a condition due to which various pathogens and toxins pass through the lining of the gut and move freely in the body, this leads to various conditions. Leaky gut syndrome, if not treated can be a life-threatening condition.
Colostrum is an optimal treatment for treating leaky gut syndrome because it has growth factors that help repair the damage of the intestine to normal. It is also rich in immunoglobulins that control the pestering of fungi and bacteria in the body. In various conducted studies colostrum has successfully increased the surface area of the lining of the intestine, thereby improving the absorption of nutrients.
Colostrum: The Perfect & Functional Food
Looking at all the immune and growth factors that are present in colostrum, it is called the best alternative to pharmaceutical drugs, from steroids and antibiotics. Colostrum is also safe for people suffering from lactose intolerance and has no allergic reactions or side effects.
A functional food is one that has potential health benefits compared to normal food and is high in nutrients. Colostrum is high in nutrients and can be combined with other food products. It is most effective when taken on an empty stomach. Available in the form of capsules, colostrum is more effective and bioavailable.
Colostrum for Autoimmune Conditions:
Autoimmune conditions are those in which the body starts producing antibodies against itself. Colostrum has shown to be highly effective to treat autoimmune conditions like Lupus, Parkinson’s disease, and Multiple Sclerosis. Chemokine receptors have been observed to be the cause of the development of all these conditions. Colostrum produces antagonists of these receptors and has been shown to decrease the symptoms of many common autoimmune conditions.
Colostrum Used as a Topical Application:
Colostrum, if applied externally can help heal the burns, acne, cuts and various abrasions and even surgical cuts. If applied orally, it can help deal with sensitive teeth relieve canker sores and gingivitis.
Some Overall Benefits of Colostrum are:
Anti-inflammatory
Anti-aging
Anti-fungal
Anti-bacterial
Cancer
AuAutoimmuneondition
Blood pressure
Cholesterol
Sugar levels
Diabetes
Digestion
Flu prevention
Fat reduction
Heart health
Gut health
Immunity
Joint repair
Immunity
Mobility
Muscle repair
Pain
Stamina
Tissue repair
Wound healing
Weight loss
Inflammation
Below is a list of some common conditions for which colostrum can be effective:
Allergies
Anemia
Arthritis
Autoimmune conditions
Asthma
Bacterial infection
Bone marrow transplant
Cancer
Alcoholism
Allergies
Anemia
Arthritis
Crohn’s disease
Chronic fatigue
Diarrhea
Fibromyalgia
Food poisoning
Heart disease
Hepatitis
Influenza
Intestinal bowel syndrome
Joint repair
Leaky gut
Lupus
Multiple sclerosis
Osteoporosis
Obesity
Premature birth
Osteoporosis
Ulcer
Yeast infection
Viral infection
Where Can I Find Colostrum?
If you have any symptoms suggestive of gastro-intestinal dysbiosis (diarrhea, constipation, bloating, reflux, stomach discomfort or pain) then you should seek further work-up by your physician or a Functional Medicine Doctor.
In the meantime, it is recommended to start using Bovine Colostrum which can be found at Sovereign Laboratories at www.mysovlabs.com. Simply mix 2 tablespoons in 6oz of water and consume twice per day on an empty stomach. This product is full of gut healing immunoglobulins. Use for 2-3 months should result in significant improvement.
In addition, it is also recommended to take a good probiotic while using your bovine colostrum. Vitamin D levels should be optimized to levels between 80-100.

by admin | Jul 24, 2018 | Health Awareness, Multiple Sclerosis, Parkinson's Disease, Rheumatoid Arthritis, Stem Cell Therapy
In a recent edition of JAMA, the results of a 30-year study examining the possible connection between stress and autoimmune disease were revealed. The findings don’t simply demonstrate a link; instead, they reveal that stress-related disorders are significantly associated with risks of developing the subsequent autoimmune disease. In the study of over 100,000 subjects, the correlation showed that individuals with a diagnosed stress-related disorder were 30-40% more likely to later be diagnosed with one of many possible autoimmune diseases.
What is a Stress-Related Disorder?
The type of stress study subjects encountered is not to be confused with the stressors we encounter during everyday life. Sitting in traffic or worrying about being late for a meeting, for example, are examples of acute stress. These forms of short-term stress generally come and go but fail to create the sort of long-term damage produced by chronic stress, or stress-related disorders.
Stress-related disorders are mental health conditions resulting from short- and long-term anxiety from mental, physical, or emotional stress. Examples of these include post-traumatic stress disorder (PTSD), obsessive-compulsive disorder, acute stress reaction, and adjustment disorder.
Which Types of Autoimmune Disorders Are Linked to Stress?
According to the study’s findings, individuals with stress-related disorders were more inclined to be diagnosed with one of 41 autoimmune disorders. Among the many autoimmune diseases observed by the research were psoriasis, Crohn’s disease, rheumatoid arthritis, and celiac disease.
Interestingly, additional variables seemed to further increase – or decrease – one’s risks of developing an autoimmune disease. Being diagnosed with PTSD at a young age, for instance, increased risks, while receiving antidepressant treatment shortly after being diagnosed with PTSD lowered rates of subsequent autoimmune disease diagnosis. Thus, it could be inferred that receiving treatment for a stress-related disorder may help to treat not only the stress itself but also minimize the lasting implications caused by it, including increased risks of disease.
What Causes the Connection?
Further research must still be conducted to pinpoint the precise long-term effects stress has on the body, and more specifically, on the immune system. Experts speculate that factors such as changes in cortisol levels and pro-inflammatory cytokine levels may need to be examined. Another hypothesis set forth by researchers is that individuals living with conditions such as PTSD might be more inclined towards unhealthy behaviors such as drinking more alcohol or sleeping less.
Although further research into this connection has yet to be conducted, one important takeaway from the findings is the fact that seeking treatment for stress-related disorders should now be considered more critical than ever. By consulting mental health professionals, individuals living with these conditions can pursue a tailored treatment approach to support short- and long-term improvements in overall wellness. For those with an auto-immune condition, see how stem cell therapy may help your symptoms and improve quality of life.

by admin | Jul 20, 2018 | Stem Cell Research, Stem Cell Therapy
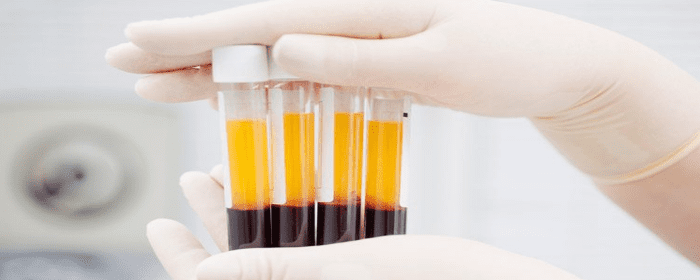
Adipose treatment is a procedure in which stem cells are derived from a section of the abdomen then used for therapeutic purposes. Adipose-derived stem cells (ADSCs) are less invasive to extract compared to cells derived from other sources, such as bone marrow. In therapeutic applications, adding platelet-rich plasma (PRP) to ADSCs has been shown to have benefits.
What is PRP?
PRP therapy is the process by which a small sample of blood is removed from the patient. The platelets are then separated from other components of the blood via a centrifuge. The isolated platelets are shown to have high levels of diverse growth factors.
Why Are Growth Factors Important?
ADSCs are shown to have reduced proliferative potential. While they do secrete a wide range of growth factors, PRP therapy is coupled with stem cell therapy to maximize their regenerative medicine potential benefits by helping to increase their proliferation and differentiation. PRP essentially empowers the ADSCs, stimulating cell proliferation and cell differentiation when used for regenerative applications.
Which Applications Can the Therapy Be Used for?
Researchers have stated that the therapeutic potential of ADSCs is “enormous,” but by kickstarting the stem cells with PRP, it’s possible that the therapy will unlock even further medicinal possibilities. Anti-inflammatory and anti-apoptotic effects have been demonstrated by ADSCs, and there are many clinical trials which have either been completed or are ongoing to explore the treatment’s effects. Skeletal repair, soft tissue generation, and immune disorders such as Crohn’s disease and multiple sclerosis are just some of the therapeutic targets for this treatment. In specific, using ADSCs with PRP has been shown to aid periodontal tissue engineering, tendon repair, wound healing, and even bone regrowth.
Because the ADSCs are fairly easy to source and blood samples required for PRP are also simple to acquire, combining PRP to adipose stem cell therapy shows promise for delivering a powerful treatment that can address a broad variety of conditions, all with a minimally invasive approach.





 St. Petersburg, Florida
St. Petersburg, Florida
