
by admin | Jun 5, 2025 | Chronic Pain, Regenerative Medicine, Stem Cell Research, Stem Cell Therapy, Studies
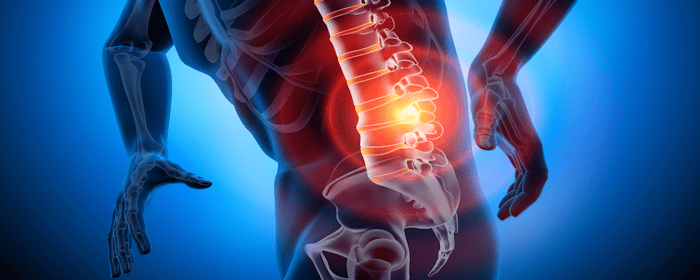
Chronic low back pain (cLBP) remains one of the most prevalent and disabling conditions worldwide, significantly affecting the quality of life and productivity for millions of individuals. For those dealing with discogenic cLBP—pain caused by degeneration or damage to the intervertebral discs—the journey toward lasting relief is often frustrating and unsuccessful. Traditional treatment options frequently fall short, prompting a growing interest in regenerative medicine.
The objective of this study was to evaluate the safety and efficacy of a novel, pragmatic algorithm that both diagnoses and treats cLBP by first identifying annulus fibrosus tears (fissures) in the region of symptoms and then delivering targeted fibrin sealant therapy to support disc repair and functional recovery.
Limitations of Traditional Treatments
Surgical options such as spinal fusions and discectomies have long been used to manage disc-related pain. However, these procedures carry inherent risks and may inadvertently accelerate degeneration. Fusions can increase stress on adjacent discs, while discectomies may weaken the disc structure, making them less than ideal for long-term relief.
Regenerative techniques such as bone marrow aspirate concentrate (BMC) and platelet-rich plasma (PRP) injections offer more conservative alternatives. Still, they lack a crucial component—adhesive properties that enable the treatment to remain within the disc and target the damaged tissue. Without a way to stay in place, these therapies often fail to promote true disc healing.
Evaluating Fibrin Sealant as a Regenerative Option
Fibrin, a natural component of the body’s wound healing process, offers unique advantages as a regenerative therapy. As a bio-adhesive, fibrin can immediately bind to tears in the annulus fibrosus—the tough outer layer of the disc—helping to stabilize the structure and promote tissue regeneration. This property makes fibrin an ideal candidate for treating chronic discogenic pain in a minimally invasive way.
A recent study explored the use of intra-annular injections of allogeneic fibrin sealant to treat patients suffering from chronic low back pain, even after multiple failed interventions. The results are compelling and suggest a promising path forward for individuals living with this difficult condition.
Study Overview and Patient Population
This retrospective cohort study involved 827 patients who had suffered from chronic low back pain—with or without radiculopathy (leg pain due to nerve irritation)—for a minimum of six months. The average duration of symptoms before treatment was 11.2 years, highlighting the severity and chronicity of the condition in the study group.
Participants had already failed at least four invasive treatments, including PRP, BMC, epidural steroid injections, and radiofrequency neurotomies. Some had even undergone failed surgeries such as discectomies and spinal fusions. The mean patient age was 56, and the cohort included 70% men and 30% women.
To qualify, patients underwent MRI and radiographic evaluations to rule out other conditions like spinal fractures, malignancies, severe instability, or significant stenosis. Only those who met strict inclusion criteria were eligible for treatment.
Diagnostic and Treatment Process
The study utilized annulargrams, an imaging technique used during the procedure to identify annular tears within the disc. These diagnostic tools were performed concurrently with the fibrin injections, providing real-time guidance to ensure precise delivery.
Allogeneic fibrin sealant was then injected into the identified fissures in the annulus fibrosus using fluoroscopic imaging to guide placement. Because fibrin adheres directly to the tissue, it integrates with the disc and creates a scaffold for tissue repair.
This innovative approach combines diagnosis and treatment in a single session, improving efficiency and potentially reducing healthcare costs. Future research may examine the cost-effectiveness of this streamlined model.
Promising Patient Outcomes
The results of the study were highly encouraging. Significant improvement was observed at one, two, and three years after the procedure across multiple outcome measures, including the:
- Oswestry Disability Index (ODI)
- Visual Analog Scale (VAS)
- PROMIS® Physical and Mental Health Scores
At the 12-month follow-up, 50% of patients achieved the minimum clinically important difference (MCID) in the ODI score, indicating meaningful functional improvement. Importantly, these positive outcomes were consistent across age, gender, comorbidity, and prior treatment history.
Interestingly, patients who had previously undergone surgery saw even greater relative improvement than those who had not. This finding suggests that fibrin injections may offer an effective second-line treatment for patients who did not benefit from surgical interventions.
Safety and Adverse Events
No severe adverse events or infections were reported in the study, which is consistent with existing literature showing low complication rates from interventional spine procedures. Fibrin’s natural bio-compatibility may contribute to its favorable safety profile.
Given the high safety margin and promising results, fibrin sealant appears to be a well-tolerated and effective treatment option for patients with discogenic low back pain.
Study Limitations and Future Directions
Despite its promising outcomes, the study was not without limitations. It was a retrospective analysis of prospectively collected data, and it lacked a randomized control group. Participants acted as their own controls through pre- and post-treatment comparisons. Additionally, the voluntary nature of treatment selection may have introduced selection bias.
The gender imbalance (30% female, 70% male) also limits the generalizability of results. Future studies should aim for more gender-balanced samples to better understand how the treatment performs across populations.
Patient compliance with follow-up assessments decreased over time, which may have affected the consistency of long-term outcome data. Still, the data were independently collected and analyzed by statisticians using validated tools from the Regenerative Orthobiologics Registry, which adds credibility to the findings.
To strengthen the evidence base, future studies should include randomized controlled trials (RCTs), longer follow-up periods, broader patient populations, and comparative studies against other biologics like PRP and BMC.
A New Path Forward in Regenerative Spine Care
For patients who have struggled with years of chronic low back pain, intra-annular fibrin sealant offers new hope. Its unique adhesive properties allow it to bind securely within the disc, facilitating healing where other treatments have failed. Even in patients with extensive treatment histories, including failed surgeries, fibrin sealant injections demonstrated the ability to restore function and relieve pain.
This study highlights the regenerative potential of harnessing the body’s own healing mechanisms in a minimally invasive way. It also sets the stage for larger clinical trials that can solidify fibrin sealant’s place in the treatment landscape for discogenic cLBP.
Fibrin Sealant Injections: A Breakthrough in Regenerative Treatment for Chronic Discogenic Back Pain
Intra-annular injections of allogeneic fibrin sealant represent a safe, effective, and innovative approach to managing chronic discogenic low back pain and radiculopathy. With improvements documented across both physical and mental health outcomes—and a favorable safety profile—this treatment offers a compelling alternative for patients who have not responded to traditional therapies.
As the field of regenerative medicine continues to evolve, treatments like fibrin sealant provide not only physical relief—but also hope—for those whose lives have been disrupted by chronic back pain.
Source: Pauza K, Boachie-Adjei K, Nguyen JT, Hussey Iv F, Sutton J, Serwaa-Sarfo A, Ercole PM, Wright C, Murrell WD. Long-term Investigation of Annulargrams and Intra-annular Fibrin to Treat Chronic Discogenic Low Back Pain and Radiculopathy: 1-, 2-, and 3-Year Outcome Comparisons of Patients with and without Prior Surgery. Pain Physician. 2024 Nov;27(8):537-553. PMID: 39621982.

by admin | May 29, 2025 | Regenerative Medicine, Stem Cell Research, Stem Cell Therapy
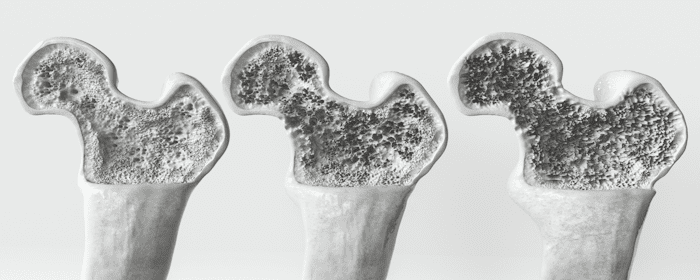
Osteoporosis is a chronic condition that causes bones to become thinner, more fragile, and more likely to break. It affects millions of people worldwide and becomes more common with age, particularly in postmenopausal women and older men. While current treatments can slow the progression of the disease, they cannot rebuild lost bone or fully reverse the damage. For this reason, regenerative medicine—specifically stem cell therapy—is emerging as a promising approach to managing and potentially treating osteoporosis.
In this review, Arjmand et al. explore how stem cells, particularly mesenchymal stem cells (MSCs), are being studied for their ability to restore bone strength and improve skeletal health in people with osteoporosis.
Understanding the Progression of Osteoporosis
Osteoporosis gradually weakens bones over time by reducing both their density and structural integrity. As bones lose mass, they become more brittle and prone to fractures. The condition is most often diagnosed in people over the age of 65, and the risk increases significantly after menopause due to a drop in estrogen levels.
The hip, spine, wrist, and shoulder are common fracture sites in individuals with osteoporosis. Among these, hip fractures are especially serious, often requiring hospitalization and long periods of rehabilitation. In some cases, these fractures can significantly reduce mobility and overall quality of life. With global life expectancy increasing, osteoporosis is becoming a major public health concern, creating both physical and economic burdens.
The Role of Bone Remodeling and How It Changes with Age
Bone tissue is constantly being broken down and rebuilt through a process known as bone remodeling. This cycle involves two types of cells: osteoclasts, which remove old or damaged bone, and osteoblasts, which generate new bone tissue. In healthy individuals, the activity of these cells is balanced, ensuring that bones remain strong and functional.
As people age, this balance often shifts. Osteoblasts become less active, producing fewer new bone cells, while osteoclasts continue breaking down old bone at a normal or accelerated rate. This leads to a gradual loss of bone mass and an increased risk of fractures.
Bone remodeling is influenced by various internal signaling molecules, including hormones, cytokines, and growth factors. These elements help regulate the function of bone cells, determining how much bone is built and how quickly it is resorbed. In people with osteoporosis, the signaling environment often favors bone loss over bone formation.
Diagnostic Tools and Treatment Limitations
Osteoporosis is most commonly diagnosed using dual-energy X-ray absorptiometry (DXA), a scan that measures bone mineral density (BMD). Results from this scan are given as T-scores, which compare a person’s bone density to that of a healthy 30-year-old adult. A T-score below -2.5 is considered diagnostic for osteoporosis, while scores between -1.0 and -2.5 indicate low bone mass, or osteopenia.
Current treatments for osteoporosis include both pharmacological and non-pharmacological approaches. Medications such as bisphosphonates, selective estrogen receptor modulators (SERMs), hormone therapy, denosumab, and parathyroid hormone analogs are commonly prescribed to reduce bone loss and lower the risk of fractures. In addition, nutritional support, physical exercise, and fall prevention strategies are recommended to help manage the condition.
Although these treatments can slow the progression of osteoporosis and reduce fracture risk, they do not reverse bone loss. Many medications also come with potential side effects, particularly when used long-term. This has created a need for more advanced and effective therapies capable of regenerating bone tissue and restoring skeletal strength.
Regenerative Medicine and the Promise of Stem Cells
In recent years, regenerative medicine has gained momentum as a potential solution for chronic diseases like osteoporosis. Stem cell therapy, in particular, offers a unique opportunity to not only halt bone loss but also encourage new bone formation. This represents a significant shift from simply managing the disease to actively repairing the damage it causes.
Mesenchymal stem cells (MSCs) are among the most studied types of stem cells for orthopedic and bone-related applications. Found in tissues such as bone marrow, adipose tissue, and umbilical cord tissue, MSCs have the ability to develop into several cell types, including osteoblasts—the bone-forming cells responsible for building new bone. These stem cells also release important signaling molecules that promote healing, reduce inflammation, and support tissue regeneration.
MSCs are considered ideal for therapeutic use because they are immune-privileged, meaning they are less likely to be rejected by the body, and they carry fewer ethical concerns compared to embryonic stem cells. Their ease of collection and expansion in the lab further enhances their clinical value.
The Mechanism of Stem Cell Action in Bone Repair
Bone formation involves two main processes: intramembranous ossification and endochondral ossification. Both rely on the activity of osteoblasts, and both can be influenced by MSCs. In intramembranous ossification, which forms flat bones such as those in the skull, MSCs directly convert into bone cells. In endochondral ossification, which forms long bones like the femur, cartilage is first created and then gradually replaced by bone. MSCs support both of these processes by providing the raw materials and signaling cues needed for effective regeneration.
In addition to becoming bone-forming cells, MSCs contribute to bone health through the release of growth factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and transforming growth factor beta (TGF-β). These molecules help coordinate the repair of damaged bone by stimulating cell growth, promoting blood vessel formation, and guiding the production of extracellular matrix—the material that gives bone its structure.
Stem cells also help rebalance the activity between osteoblasts and osteoclasts. In osteoporosis, where bone breakdown exceeds bone formation, MSCs can shift the system back toward growth by reducing osteoclast activity and encouraging osteoblast development. This dual action makes stem cell therapy particularly appealing as a comprehensive treatment for osteoporosis.
Emerging Therapies and Scientific Insights
Recent studies in animals and early-stage human trials have shown promising results for the use of MSCs in treating osteoporosis. These studies report improvements in bone density, strength, and healing time. In laboratory settings, MSCs have been shown to stimulate the growth of bone tissue and reduce inflammation, which is often elevated in individuals with bone loss.
Another emerging area of interest involves MSC-derived exosomes. These are tiny particles released by stem cells that carry the same regenerative molecules found in the cells themselves. Because exosomes can be isolated and administered without the need for transplanting whole cells, they offer a potentially safer and more targeted method of treatment.
The use of exosomes could allow for new forms of therapy that minimize immune reactions, simplify storage and transport, and avoid some of the challenges associated with live cell therapies. Early research suggests that exosome-based treatments may support bone remodeling, enhance blood supply, and reduce the severity of osteoporosis-related damage.
Future Directions in Osteoporosis Treatment
As regenerative medicine continues to evolve, future therapies for osteoporosis may include personalized stem cell treatments tailored to an individual’s genetic profile and bone health history. Scientists are also exploring the use of medications like oxytocin and parathyroid hormone to activate the body’s own stem cells and encourage bone regeneration from within.
Advancements in areas such as metabolomics—the study of small molecules in biological systems—may improve diagnosis and treatment selection for people at risk of osteoporosis. This could allow healthcare providers to better understand how a person’s metabolism affects bone loss and response to therapy.
Personalized medicine, which involves designing treatments based on an individual’s specific biological characteristics, is likely to play an increasing role in osteoporosis care. By combining genetic insights with regenerative therapies, it may be possible to offer more effective, targeted solutions for bone repair and long-term skeletal health.
Moving Toward Restoring Bone Health
Osteoporosis remains a significant health concern, particularly as the global population continues to age. While current treatments can slow its progression, they do not address the root cause of bone loss. Regenerative medicine offers a transformative path forward by focusing on rebuilding bone tissue rather than simply preserving what remains.
Mesenchymal stem cells have emerged as a leading option in the development of osteoporosis therapies. Their ability to both create new bone and modulate the biological environment around them makes them a powerful tool in the quest to restore bone health. Although more research is needed to refine treatment protocols and ensure safety, the future of stem cell therapy for osteoporosis is full of potential.
Source:
Arjmand B, Sarvari M, Alavi-Moghadam S, Payab M, Goodarzi P, Gilany K, Mehrdad N, Larijani B. Prospect of Stem Cell Therapy and Regenerative Medicine in Osteoporosis. Front Endocrinol (Lausanne). 2020 Jul 3;11:430. doi: 10.3389/fendo.2020.00430. PMID: 32719657; PMCID: PMC7347755.

by admin | May 27, 2025 | Regenerative Medicine, Spinal Cord Injury, Stem Cell Research, Stem Cell Therapy
Spinal cord injuries (SCIs) can have devastating effects on a person’s mobility, independence, and quality of life. Each year, thousands of individuals experience life-altering damage to the spinal cord that results in partial or total paralysis, chronic pain, and long-term health complications. These injuries place a tremendous emotional and economic burden on patients, families, and healthcare systems around the world.
Despite advances in emergency care and rehabilitation, current treatment options for SCIs remain limited in their ability to restore lost function. Traditional interventions often focus on managing symptoms or preventing further damage rather than reversing the injury. However, a promising area of research is emerging in the field of regenerative medicine.
As part of this review, Zeng et al. provide a comprehensive overview of the current state of stem cell therapy for spinal cord injuries, examining various stem cell types, their therapeutic potential and limitations, key challenges in clinical application, and emerging technologies aimed at advancing regenerative treatment outcomes.
The Role of Stem Cells in Spinal Cord Repair
Stem cells are unique because they can transform into different types of cells in the body. In the case of spinal cord injuries, researchers are exploring how stem cells might replace damaged neurons, regenerate supportive tissue, and promote healing in the central nervous system. Stem cells may also help regulate inflammation, improve the local environment for nerve growth, and protect existing cells from further damage.
There are several types of stem cells being studied for their potential in spinal cord repair. Embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem or progenitor cells (NSPCs) each offer different benefits and challenges. Some types are better at creating new neurons, while others are more effective at reducing inflammation or supporting the overall healing process.
ESCs are derived from early-stage embryos and have the remarkable ability to become any cell type in the body. However, their use raises ethical questions, and they carry a risk of forming tumors after transplantation. iPSCs are created by reprogramming adult cells to behave like embryonic stem cells. These cells avoid many of the ethical concerns associated with ESCs, but they still present risks related to tumor formation and genetic instability.
MSCs, typically harvested from bone marrow, fat, or umbilical cord tissue, have shown promise in reducing inflammation and promoting repair. They are relatively easy to collect and prepare for therapeutic use. NSPCs are naturally present in the nervous system and have the potential to become various types of neural cells. They offer a more targeted approach for regenerating nervous tissue but are still being refined for clinical use.
Evidence from Laboratory and Clinical Studies
Extensive laboratory research has shown encouraging results using stem cell therapies in animal models of spinal cord injury. In these studies, stem cell transplantation has led to improvements in movement, reduced scar formation, and enhanced nerve regeneration. Researchers have observed that transplanted cells can form new connections, produce growth factors that support healing, and modulate the immune response to minimize further damage.
Some early-stage clinical trials have begun to translate these findings into treatments for human patients. While results have been mixed, a number of studies have reported meaningful improvements in motor function, bladder control, and sensory perception. These gains, although modest in some cases, suggest that stem cell therapy could be a powerful tool in the future management of SCIs.
The authors note that most stem cell therapies are still in the experimental phase and call for long-term studies to better understand the safety, effectiveness, and best practices for delivering these treatments. Nonetheless, the growing body of evidence is an encouraging sign that stem cells may play a major role in future treatment strategies.
Enhancing Stem Cell Therapy with New Technologies
To improve the effectiveness of stem cell treatments, researchers are combining them with advanced technologies such as biomaterials, gene editing, and tissue engineering. One area of focus is the use of scaffolds—biocompatible materials that provide a physical structure to support cell survival and guide the growth of new tissue. These scaffolds can be custom-designed using 3D printing techniques to fit the exact shape of a patient’s injury site.
Gene editing tools, such as CRISPR-Cas9, are also being explored to enhance the therapeutic potential of stem cells. Scientists are investigating ways to modify stem cells before transplantation to increase their ability to survive in the hostile environment of an injured spinal cord or to encourage them to differentiate into specific types of cells that are most needed for recovery.
Tissue engineering strategies involve creating complex structures that mimic the architecture of the spinal cord. These engineered tissues can be used to deliver stem cells in a controlled and effective manner, ensuring they integrate properly with existing neural circuits and contribute to long-term healing.
Challenges to Overcome in Clinical Application
A major challenge is determining the best timing for stem cell transplantation. The spinal cord undergoes a complex series of changes in the weeks following an injury, including inflammation, scar formation, and cell death. Transplanting stem cells too early may expose them to a highly toxic environment, while waiting too long may limit their ability to integrate effectively. Finding the ideal therapeutic window is a key area of ongoing research.
Standardizing stem cell protocols is also essential. Differences in how cells are harvested, processed, and administered can affect outcomes and make it difficult to compare results across studies. Establishing clear guidelines for manufacturing and delivering stem cell therapies will be crucial for advancing them into mainstream clinical use.
Moving Toward Personalized Treatment Approaches
One of the most exciting aspects of stem cell therapy is the potential for personalized medicine. Because stem cells can be derived from a patient’s own tissues, it may be possible to create custom treatments that are less likely to be rejected by the immune system. Personalized approaches could also involve selecting the most appropriate cell type or combination of therapies based on a patient’s specific injury characteristics and recovery goals.
Researchers are also developing better tools for monitoring treatment success. Advanced imaging techniques, biomarker analysis, and functional assessments can help track how well stem cells are working and guide adjustments in therapy. These tools are essential for evaluating progress and ensuring that patients receive the most effective and appropriate care.
A Hopeful Future for Spinal Cord Injury Treatment
Although still in the early stages of stem cell therapy for spinal cord injuries, the progress so far is encouraging. With ongoing research, technological innovation, and clinical testing, it is likely that stem cells will play a major role in transforming how SCIs are treated in the future. These therapies offer hope not only for repairing the damage caused by injury, but also for restoring independence, mobility, and quality of life for those affected.
Zeng et al conclude that the journey ahead will involve addressing technical and ethical challenges, refining treatment protocols, and ensuring that therapies are safe, effective, and accessible. But with continued dedication from the global scientific community, the promise of regenerative medicine may one day become a reality for patients living with spinal cord injuries.
Zeng CW. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int J Mol Sci. 2023 Sep 20;24(18):14349. doi: 10.3390/ijms241814349. PMID: 37762654; PMCID: PMC10532158.

by admin | May 22, 2025 | Health Awareness
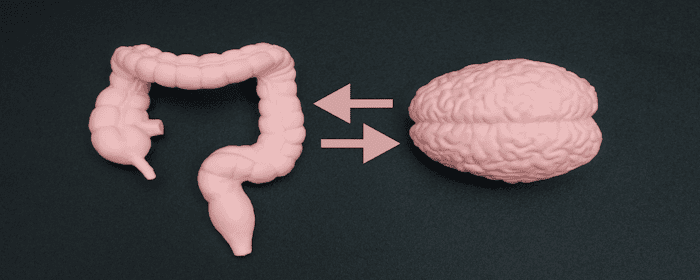
We’ve all heard the phrase “trust your gut,” but did you know your gut health is closely tied to your mood, energy, and even how you think? More and more research is showing that the gut and brain are deeply connected, and keeping your digestive system healthy can make a big difference in your overall mental wellness.
The Gut-Brain Connection
Your digestive system is sometimes called your “second brain” because it has its own network of nerves, known as the enteric nervous system. This system communicates constantly with your brain through the gut-brain axis.
Inside your gut live trillions of bacteria and microorganisms, often referred to as the gut microbiome. These tiny organisms help with digestion, immune support, and even the production of neurotransmitters like serotonin and dopamine, which influence mood, sleep, and motivation.
When the gut microbiome is balanced, it supports mental clarity, stable moods, and resilience to stress. But when it’s out of balance, it can contribute to issues like:
- Anxiety and stress
- Low mood or depression
- Brain fog and poor concentration
- Trouble sleeping
What Harms Gut Health?
Several everyday factors can disrupt gut balance:
- A diet high in processed foods and sugar
- Frequent antibiotic use
- Chronic stress
- Poor sleep
- Lack of fiber and nutrient-rich foods
When these factors pile up, it can lead to inflammation, which not only affects digestion but also your brain and nervous system.
How to Support Gut and Mental Wellness
The good news is that small, consistent steps can help improve both gut health and mood:
- Eat a diverse diet rich in fiber, vegetables, fruits, lean proteins, and fermented foods like yogurt, kefir, or sauerkraut.
- Manage stress with relaxation techniques such as meditation, yoga, or deep breathing.
- Stay hydrated to support digestion and nutrient absorption.
- Prioritize sleep, since rest is when the gut lining and brain both repair themselves.
- Consider nutrient support — vitamins and minerals play a big role in gut and brain health.
How Our Clinic Can Help
At Stemedix, we understand that whole-body wellness includes both physical and mental health. We offer therapies designed to restore balance, reduce inflammation, and give your body the nutrients it needs to thrive.
- Vitamin Infusions: Targeted blends like the Myer’s Cocktail can replenish essential nutrients that support both digestion and mood regulation.
- Regenerative Therapies: By addressing inflammation and supporting the body’s repair systems, these treatments may help restore balance from the inside out.
Taking care of your gut isn’t just about digestion, it’s about supporting your brain, your mood, and your overall well-being.
Overall Takeaway
A healthy gut means a healthier mind. If you’ve been struggling with fatigue, stress, or mood swings, it may be time to look deeper into your gut health.
Contact us today to learn how our wellness therapies can help restore balance and support both your gut and your mind.

by admin | May 20, 2025 | COPD, Regenerative Medicine, Stem Cell Research, Stem Cell Therapy
Chronic obstructive pulmonary disease, or COPD, is a long-term lung disease that causes ongoing inflammation and irreversible damage to lung tissues. This damage affects both the structure and function of the lungs, leading to serious breathing problems. COPD is a major health challenge worldwide, causing significant illness and death. Although current treatments can ease symptoms, they do not repair the damage that COPD causes to the lungs.
Because of these limitations, researchers have turned to regenerative medicine and the exciting potential of stem cell therapy to find better treatments. Stem cells are special cells with the ability to renew themselves and transform into different types of cells. Scientists are exploring how stem cells could help repair damaged lung tissue and improve lung function in people living with COPD.
How COPD Develops and Damages the Lungs Over Time
COPD is caused mainly by long-term exposure to harmful substances such as cigarette smoke, air pollution, and certain chemicals. In some cases, a genetic condition called α1-antitrypsin deficiency can also increase the risk. These factors lead to chronic inflammation, destruction of lung tissue, and narrowing of the airways.
Two common forms of COPD are chronic bronchitis and emphysema. Chronic bronchitis involves inflammation of the lining of the airways, which leads to excessive mucus production and swelling. This mucus buildup blocks airflow, making it difficult to breathe. Emphysema, on the other hand, damages the tiny air sacs called alveoli, which are essential for oxygen exchange. This damage causes the air sacs to enlarge and lose elasticity, reducing the lungs’ ability to transfer oxygen into the bloodstream.
As COPD worsens, patients experience increasing difficulty in breathing, often feeling breathless even during mild activity. This progressive lung damage also leads to other complications like airway hyperresponsiveness and overlapping lung diseases.
Current Treatments and Their Limitations
Today, there is no cure for COPD. The main treatments focus on controlling symptoms, reducing inflammation, and improving quality of life. Quitting smoking is the most important step to slow disease progression. Other treatments include medications such as bronchodilators and steroids, oxygen therapy, vaccinations to prevent infections, and pulmonary rehabilitation to help patients breathe better.
While these treatments can relieve symptoms and improve lung function temporarily, they do not stop or reverse the underlying damage to lung tissue. This means the disease continues to progress over time despite therapy. Because of this, scientists are urgently seeking new approaches that can restore lung function by repairing or regenerating damaged lung tissues.
Stem Cells: A Promising Avenue for Lung Repair
Stem cells are unique cells capable of dividing endlessly and turning into different types of mature cells. This remarkable ability makes them an ideal candidate for regenerative medicine, which aims to heal damaged organs and tissues. In COPD, stem cells might be able to replace destroyed lung cells, reduce inflammation, and promote the natural repair process.
There are several types of stem cells under investigation for COPD treatment. Embryonic stem cells (ESCs) are derived from early-stage embryos and can develop into almost any cell type. Induced pluripotent stem cells (iPSCs) are adult cells reprogrammed to an embryonic-like state, also able to become many different cell types. Adult stem cells exist in various tissues and serve as the body’s repair system. Among adult stem cells, mesenchymal stem cells (MSCs) are widely studied for lung repair.
Comparing Different Stem Cell Types
Lung progenitor cells are specialized to the lungs but are rare and difficult to obtain. MSCs, which can be harvested from bone marrow, fat tissue, and other sources, are easier to collect and have lower chances of immune rejection and tumor formation. MSCs also have strong anti-inflammatory properties, making them attractive for treating inflammatory lung diseases like COPD.
Despite these advantages, MSCs have some challenges, such as variability in their behavior and the risk of aging or senescence, which could limit their effectiveness. Researchers continue to study ways to enhance the safety and efficacy of MSC-based treatments, including combining them with other therapies or using supportive materials that help stem cells survive and integrate into lung tissue.
How Mesenchymal Stem Cells Help Repair Lung Damage
MSCs have been tested in animal models of lung injury with encouraging results. They appear to help repair lung tissue by several mechanisms. One is cell replacement: MSCs can transform into lung-specific cells and replace damaged cells, improving the lung’s structure and function. Another way is through paracrine effects, meaning MSCs release various substances that encourage the body’s own repair systems.
Studies show that when MSCs are introduced into the lungs, they do not simply settle there in large numbers but instead release molecules that reduce inflammation, attract native stem cells, and stimulate regeneration. These molecules include anti-inflammatory factors and growth factors that help heal damaged tissue and prevent cell death.
In animal models, MSC treatment has reduced lung damage caused by cigarette smoke and improved lung function. MSC-derived secretions, like conditioned medium (the fluid containing MSC-released factors) and extracellular vesicles (tiny particles carrying proteins and genetic material), have also shown protective and reparative effects in lung injury studies. These findings suggest that MSCs help repair lung tissue both by becoming new lung cells and by signaling the body to heal itself.
What Stem Cell Advances Mean for COPD Treatment
While the research on stem cell therapies for COPD is still largely in the preclinical stage, it holds great promise for the future. MSCs in particular offer a potentially safe and effective approach to slow down, stop, or even reverse lung damage. Future treatments might involve infusions of MSCs, the use of MSC secretions, or combinations with other treatments to maximize lung repair.
Scientists are also exploring ways to improve stem cell therapies, such as by pre-conditioning MSCs before transplanting them or combining them with gene therapy. New techniques involving 3D scaffolds and biomaterials might help stem cells survive and work better inside damaged lungs.
A New Frontier in COPD Treatment
COPD remains a serious and progressive disease with limited treatment options. Although current therapies manage symptoms, they do not restore lost lung function. Regenerative medicine and stem cell therapy, especially using mesenchymal stem cells, represent a hopeful new direction. These therapies aim to repair lung damage and improve lung function by leveraging the natural ability of stem cells to regenerate tissue and reduce inflammation.
Continued research and clinical trials are essential to fully understand how best to use stem cells for COPD and to ensure these treatments are safe and effective. The day when stem cell therapy becomes a standard treatment for COPD may be on the horizon, potentially offering relief and improved quality of life for millions of patients worldwide.
Source: Lai, S., Guo, Z. Stem cell therapies for chronic obstructive pulmonary disease: mesenchymal stem cells as a promising treatment option. Stem Cell Res Ther 15, 312 (2024). https://doi.org/10.1186/s13287-024-03940-9



 St. Petersburg, Florida
St. Petersburg, Florida
