
by Stemedix | Apr 6, 2020 | Stem Cell Therapy, Chronic Pain
One in five adults in the U.S. reports having chronic pain, which amounts to 50 million people. As one of the most common reasons people visit their doctors, this frustrating symptom is linked to issues like restricted daily activities, dependence on prescription medications, anxiety and depression, and overall reduced quality of life. It can stem from many causes, including chronic conditions and lingering effects of previous injuries, and often intensifies with advancing age. Some of the most common conditions linked to chronic pain include arthritis, lupus, and multiple sclerosis. Alternative Treatment for Chronic Pain: Stem Cell Therapy.
Traditional treatments for chronic pain include over-the-counter pain relievers or opioids, along with non-medication techniques such as physical therapy, acupuncture, and exercise. Unfortunately, many patients find that the side effects of long-term pain medication can further diminish their quality of life, and other treatments often fall short when it comes to providing relief. It’s therefore no surprise that many of these patients willingly embrace new treatments, such as stem cell therapy.
Stem Cell Therapy as an Alternative Treatment for Chronic Pain
Stem cell therapy is a new form of regenerative medicine which uses the body’s natural regenerative healing mechanisms, stem cells, to repair and renew damaged tissue. These cells have the ability to self-renew and differentiate into other cell types. They also have immune-modulatory and anti-inflammatory properties, which can help address the swelling and discomfort that arises in many chronic conditions and injuries. Research suggests that their ability to home in on affected sites makes them a powerful means of treating inflammatory pain and trauma.
In patients with chronic pain, stem cells can be injected at appropriate sites to rebuild and regenerate tissue, suppress inflammation, and reduce the severity of symptoms. The treatment has been used for neurodegenerative conditions such as Parkinson’s disease and multiple sclerosis, autoimmune disorders such as rheumatoid arthritis and lupus, and musculoskeletal injuries.
Although many chronic conditions still have no cure, stem cell therapy is a promising new alternative for treating them. It goes beyond simply masking symptoms to spur healing at a cellular level, thereby leading to stronger outcomes that can significantly improve the way of life for patients experiencing chronic pain. If stem cell therapy is something you are interested in then contact us today for a free consultation.

by Stemedix | Mar 30, 2020 | Stem Cell Therapy, Musculoskeletal, Osteoarthritis
Often caused by the natural wear and tear on the joints that occurs with age, osteoarthritis occurs in millions of people throughout the U.S. and typically develops during or after an individual’s middle ages. While the condition may develop in any joint, it’s particularly common in weight-bearing joints, including the hips. Stem Cell therapy can be an option but How effective is Stem Cell Therapy for hips?
At its best, osteoarthritis in the hip may cause mild discomfort and stiffness. At its worst, it can make daily activities like tying your shoes or taking even short walks near-impossible. Unfortunately, there is currently no cure for osteoarthritis, and treatments such as invasive surgeries come with the risk of complications and long recovery periods.
Now, however, there is a new treatment available to help treat osteoarthritis and other challenging hip issues such as labral tears and bursitis: stem cell therapy for hips.
What Is Regenerative Medicine for Hips?
Stem cells have natural healing properties, along with the unique ability to self-renew and mature into specialized cell types. When strategically injected at the site of the damaged tissue, such as the hip, stem cells can trigger the body’s natural healing processes. As a result, the therapy can restore tissue, relieve inflammation and pain, and enhance mobility — even in injuries or conditions which have responded poorly to prior treatments.
How Effective Is It?
Stem cell therapy has been deemed a simple, affordable, and quick treatment for osteoarthritis of the hip. It’s also minimally invasive and improves a number of key symptoms, including pain, stiffness, and physical function. According to research, injections of mesenchymal stem cell (derived from fat tissue) have significantly improved clinical scores in patients. Results indicate that the regenerative effects of stem cells begin to take hold within two to six weeks.
Osteoarthritis isn’t the only hip condition for which stem cell therapy can be administered, however. Physicians have also been leveraging stem cell treatments in place of hip replacement surgery for conditions such as osteonecrosis. Experts believe the therapy could delay or potentially even eliminate the need for hip replacements in some cases. At the Mayo Clinic, for instance, stem cell treatment has slowed the progression of osteonecrosis by at least two to five years in 80% of patients.
In studies, patients who have received stem cell injections to alleviate hip pain have shown up to a 94% overall improvement in their condition. Reduced pain, improved flexibility, and better sleep were just a few of the results reported by participants. Studies also indicate no treatment-related adverse effects, suggesting that this form of regenerative medicine is safe and well-tolerated by patients.
If you are tired of dealing with the pain associated with your daily activities and you would like to benefit from Stem Cell therapy for the hips then Contact us today!

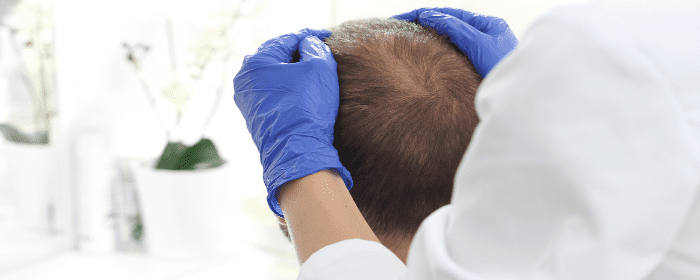
by Stemedix | Mar 24, 2020 | Hair Regrowth, Stem Cell Therapy
Considering Stem Cell Hair Restoration? Florida Residents, Find Out More
If you’re experiencing hair loss, you’re not alone: more than 50 million men and women have some degree of hair loss, which is often triggered by the natural process of aging. It can also be caused by certain illnesses and medical treatments. Thus, thinning hair is often viewed as an inevitable fact of life—but it doesn’t have to be.
If you’re considering stem cell hair restoration, a Florida-based regenerative medicine therapy provider, Stemedix, can help. They use stem cell treatments to address not only medical conditions such as neurodegenerative and autoimmune disorders, but also to promote overall wellness, which includes addressing common cosmetic concerns.
Hair loss isn’t strictly cosmetic, however. It can profoundly impact your quality of life, confidence, and satisfaction with your appearance. Over time, these negative impacts can transcend into your personal and professional relationships, taking their toll on your overall quality of life and potentially leading to larger psychological issues such as depression and anxiety.
Stem Cell Hair Restoration: Florida-Based Treatment
Fortunately, stem cell hair restoration is available for individuals who feel that hair loss or thinning is beginning to leave its mark on their lives. Whether you’ve tried medications in the past without any success or you want to avoid other interventions altogether, stem cell treatment is a promising option.
At our Florida facility, patients can receive a powerful dose of stem cell exosomes, which have hundreds of proteins and spark regeneration. Known for their ability to promote cell survival, exosomes have been shown to induce hair growth by stimulating follicles. They work by acting on several key mechanisms, including the expression of signaling molecules, to activate regenerative factors.
Patients have achieved noticeable new growth after a single treatment, though additional sessions may be needed depending on the degree of hair loss. Unlike other therapies, however, stem cell hair restoration has few to no side effects and does not require downtime or extensive healing (as seen in invasive surgical procedures). Instead, it simply turbocharges the body’s own natural regenerative processes to restore its youthful qualities. If you are looking to benefit from Stem Cell therapy then contact us today!

by Stemedix | Mar 17, 2020 | Stem Cell Therapy, Hair Regrowth
If you’ve been experiencing hair loss, you may be considering your treatment options. Many individuals have little success with widely available products that promise hair regrowth, and understandably want to avoid invasive procedures such as hair transplantation. Fortunately, there’s another option: stem cell hair restoration.
What Is Stem Cell Hair Restoration?
Stem cell therapy for hair restoration employs the use of stem cell exosomes, powerful components with an important role in cellular communication. They have over 200 signaling proteins and can turn on regenerative factors, prompting the cellular survival that can help to prevent further hair loss and stimulate new growth. Patients often notice new hair growth after just one session, with additional treatments leading to even better results.
How Much Does it Cost?
Stemedix offers stem cell therapy for hair restoration starting at $3,900. When you consider the cost of hair transplant surgeries, which can vary from $4,000 to $15,000, stem cell hair treatments become an attractive, cost-effective option. This is especially true considering the fact that, as with any surgery, recovery from hair transplants may come with additional costs, including pain medications and the cost of treating infections (the most common complication of the treatment).
Moreover, you simply can’t put a price tag on the boosted confidence, comfort, and overall improved quality of life that comes with thicker, fuller hair. Whether you’ve dodged social events or you’re experiencing low self-esteem, stem cell hair restoration could be the solution that allows you to feel like yourself once again. Hair loss has even been linked to higher levels of stress, anxiety, depression, and social phobias. Thus, finding an effective solution to restore your hair growth is about much more than cosmetics alone, and it could be the very factor that allows you to embrace a new chapter of your life.
If you find yourself noticing early signs of hair loss and you would like to reap the benefits of preventive stem cell measures contact us today for more information!

by Stemedix | Mar 10, 2020 | Stem Cell Therapy
Stem cell therapy is a promising and powerful treatment being used for a wide range of medical conditions, including orthopedic injuries and neurodegenerative diseases. With its ability to promote cellular regeneration, it has now also emerged as a treatment for cosmetic- and wellness-related issues, including hair loss. Discover more about how stem cell hair restoration works below.
Stimulating Growth with Stem Cells
Stem cell hair restoration is a regenerative medicine therapy that uses stem cell exosomes to stimulate hair follicles and activate new hair growth. Exosomes are components of stem cells which enable them to transfer genetic information to other cells within damaged tissue, such as scalp tissue with thinning hair or thwarted hair growth. They foster cell-to-cell communication, secreting more than 200 signaling proteins and sending messages to cells to promote survival and regeneration.
During the stem cell hair restoration process, exosomes are distributed to the target areas via injection to stimulate hair regrowth. The exosomes use three key mechanisms to promote hair restoration: mitochondrial dynamics, the expression of signaling molecules, and modified gene expression (epigenetics). After the stem cell injection, patients can typically resume normal routines and there is no significant downtime as seen in hair transplantation surgery, and the risk of any adverse effects is minimal.
The results from stem cell hair regeneration therapy have shown remarkable promise. Researchers call the treatment “highly effective,” and even more than four months after treatment, patients experience a significant increase in hair density. Many patients begin to see noticeable results after a single session, although multiple treatments may be needed depending on the level of hair loss.
Hair loss can be disheartening and have pronounced effects on an individual’s social and professional relationships, as well as their mental and emotional wellbeing. With this cutting-edge therapy, patients no longer have to consider ineffective products and major surgeries as their only option for fighting back against thinning hair. As an effective new alternative, stem cell hair restoration is changing the way we address hair loss and allowing individuals to regain their confidence.
If Stem Cell Hair restoration sounds like something you are interested in seeing results from Contact us today to set up your free consultation!

by Stemedix | Feb 25, 2020 | Stem Cell Therapy
For millions of people across the globe, orthopedic issues cause discomfort, limited mobility, and other frustrating symptoms which make it challenging to participate in daily activities. Osteoarthritis, for example, affects the joints by wearing away the cushiony cartilage between bones. While the prevalence of the condition is on the rise, conventional treatments are still lacking: patients are often required to choose between temporary fixes such as medications to mask the pain or invasive surgical procedures. And, this isn’t isolated to osteoarthritis; patients with other orthopedic issues face similar dilemmas. For those suffering, there are several benefits of stem cell therapy for orthopedic issues.
This treatment involves the targeted administration of stem cells, which can self-renew and transform into virtually any cell type in the body. Researchers believe they hold tremendous healing potential for orthopedic issues due to their abilities to change into cartilage cells, minimize inflammation, and release cytokines to reduce pain and slow the degenerative process.
Below, discover some of the benefits of stem cell therapy for orthopedic issues, as well as the types of conditions they can treat.
Common Orthopedic Conditions Treated with Stem Cell Therapy
The potential applications for stem cell therapy span far and wide. Not only have they been used to treat orthopedic conditions, but they’re also used for patients with neurodegenerative and autoimmune conditions.
Some of the specific orthopedic issues being treated by stem cell therapy include:
· Osteoarthritis
· Sports and athletic injuries
· Musculoskeletal injuries
· Spinal cord injuries
· Degenerative disc disease
Benefits of Stem Cell Therapy for Orthopedic Issues
There are many advantages to stem cell therapy. Here are just a few of the most noteworthy benefits to consider:
· Reduced pain and inflammation
· The ability to delay or prevent the need for invasive surgery
· Increased healing potential
· An all-natural treatment
· Safe and effective
Finally, but perhaps most importantly, this regenerative therapy can give new hope to patients who haven’t seen results from other treatment methods. Stem cell treatment could help to restore mobility, alleviate pain, reduce inflammation, and enhance overall quality of life — outcomes which may have previously seemed unattainable to patients with orthopedic issues.






 St. Petersburg, Florida
St. Petersburg, Florida
