
by admin | Nov 7, 2019 | Stem Cell Research, Adipose, Stem Cell Therapy
Osteoporosis is a disease in which bones become weak, brittle, and are prone to fracture. While osteoporosis is commonly considered a disease of low bone density, it is more complex and extensive than that. New bone is constantly formed and destroyed (resorbed) throughout life. In osteoporosis, however, the rate at which it is resorbed accelerates, while the rate at which it is formed slows down. In other words, bone is being destroyed faster than it can be formed. This process changes the size and shape of bones and alters its microarchitecture (i.e. the structure of bone on a microscopic level).
Without screening, most people will not know that they have osteoporosis until they have a bone fracture. Bones simply get weaker until some minor trauma causes one or more bones to break. Fortunately, efforts to screen for the disease (e.g. DXA/DEXA or bone density scans) have helped doctors diagnose cases of osteoporosis before the disease progresses to the point of bone fracture.
The main treatment for osteoporosis is a class of drugs called bisphosphonates. Bisphosphonates block the cells that resorb bone (osteoclasts) to allow the cells that form new bone (osteoblasts) to catch up. While bisphosphonates are effective, many patients experience severe GI side effects from these drugs including reflux, esophagitis, and ulcers, and cannot take them.
In an effort to find new ways to treat osteoporosis and help patients who cannot tolerate bisphosphonates, researchers are exploring the possibility of using stem cells to treat the disease. Ideally, one would take stem cells from patients to help regrow bone. What has been unclear was whether a person with osteoporosis still has enough healthy stem cells to effectively regrow bone.
To test this, Dr. Jiang and colleagues collected stem cells from the fat tissue of patients with osteoporosis (i.e. adipose-derived stem cells). The researchers took these stem cells and encouraged them to grow and multiply for 14 days. After the stem cells had proliferated, they injected the cells into mice and studied the effects on bone growth. After 4 weeks, the researchers saw evidence on X-ray scans that adipose-derived stem cells caused new bone growth.
These results demonstrate that even patients with osteoporosis still possess stem cells that can be used to treat their own osteoporosis. While the stem cells need to be treated in a laboratory setting for 14 days, it is potentially possible to use a patient’s own stem cells to regrow bone and treat their osteoporosis.
The next phase of research will be to conduct a clinical trial to show test whether autologous stem cell treatment (injecting a patient with their own stem cells) can regrow bone in humans. While those clinical studies will be critical in determining whether this approach is practical and effective for patients, this laboratory research is very promising.
Contact us today!
Reference: Jiang M. et al. (2014). Bone formation in adipose-derived stem cells isolated from elderly patients with osteoporosis: a preliminary study. Cell Biology International. 2014 Jan;38(1):97-105.

by admin | Oct 25, 2019 | Adipose, Osteoarthritis, Stem Cell Research, Stem Cell Therapy
Osteoporosis is a disease in which bones become weak, brittle, and are prone to fracture. While osteoporosis is commonly considered a disease of low bone density, it is actually more complex and extensive than that. New bone is constantly formed and destroyed (resorbed) throughout life. In osteoporosis, however, the rate at which it is resorbed accelerates, while the rate at which it is formed slows down. In other words, bone is being destroyed faster than it can be formed. This process changes the size and shape of bones and alters its microarchitecture (i.e. the structure of bone on a microscopic level).
Without screening, most people will not know that they have osteoporosis until they have a bone fracture. Bones simply get weaker until some minor trauma causes one or more bones to break. Fortunately, efforts to screen for the disease (e.g. DXA/DEXA or bone density scans) have helped doctors diagnose cases of osteoporosis before the disease progresses to the point of bone fracture.
The main treatment for osteoporosis is a class of drugs called bisphosphonates. Bisphosphonates block the cells that resorb bone (osteoclasts) to allow the cells that form new bone (osteoblasts) to catch up. While bisphosphonates are effective, many patients experience severe GI side effects from these drugs including reflux, esophagitis, and ulcers, and cannot take them.
In an effort to find new ways to treat osteoporosis and help patients who cannot tolerate bisphosphonates, researchers are exploring the possibility of using stem cells to treat the disease. Ideally, one would take stem cells from patients, purify them, get the cells to multiply in the lab, and inject them back into patients with osteoporosis to help regrow bone. What has been unclear was whether a person with osteoporosis still has enough healthy stem cells to effectively regrow bone.
To test this, Dr. Jiang and colleagues collected stem cells from fat tissue of patients with osteoporosis (i.e. adipose-derived stem cells). The researchers took these stem cells and encouraged them to grow and multiply for 14 days. After the stem cells had proliferated, they injected the cells into mice and studied the effects on bone growth. After 4 weeks, the researchers saw evidence on X-ray scans that adipose-derived stem cells caused new bone growth.
These results demonstrate that even patients with osteoporosis still possess stem cells that can be used to treat their own osteoporosis. While the stem cells need to be treated in a laboratory setting for 14 days, it is potentially possible to use a patient’s own stem cells to regrow bone and treat their osteoporosis.
The next phase of research will be to conduct a clinical trial to show test whether autologous stem cell treatment (injecting a patient with their own stem cells) can regrow bone in humans. While those clinical studies will be critical in determining whether this approach is practical and effective for patients, this laboratory research is very promising.
Reference: Jiang, M. et al. (2014). Bone formation in adipose-derived stem cells isolated from elderly patients with osteoporosis: a preliminary study. Cell Biology International. 2014 Jan;38(1):97-105.

by admin | Oct 18, 2019 | Adipose, Erectile Dysfunction, Stem Cell Research, Stem Cell Therapy
Prostate cancer is quite common among men in the United States. The main treatment options for prostate cancer include:
- External beam radiation – Radiation is applied to the prostate gland through the skin (noninvasive)
- Brachytherapy – Radioactive pellets the size of grains of rice are placed within the prostate gland (invasive)
- Radical prostatectomy – The entire prostate gland and some surrounding tissue is removed
About one-quarter of all men with prostate cancer ultimately choose to have a radical prostatectomy. Unfortunately, this procedure often leaves men with chronic problems afterward, such as urinary incontinence (i.e., the inability to hold or control urine) and erectile dysfunction (i.e., the inability to achieve and maintain a penile erection suitable for sexual intercourse). Almost 90% of men who undergo radical prostatectomy to treat prostate cancer develop erectile dysfunction. Drugs and penile injections are not always effective in treating this type of erectile dysfunction. Consequently, as many as three-quarters of men must live with permanent erectile dysfunction. While prostate cancer is essentially cured after radical prostatectomy, affected men have substantially worse quality of life, which also negatively affects their sexual partners.
In an effort to combat this difficult problem, researchers conducted a Phase 1 clinical trial in which they took stem cells from the patient’s own fat tissue (autologous stem cells), purified them, and injected them into the penile tissue of radical prostatectomy patients with erectile dysfunction. Eight of the 17 men who volunteered for the clinical trial regained erectile function and were able to engage in sexual intercourse after just one stem cell injection.
Importantly, stem cell treatment was only effective for men who had not developed urinary incontinence. Eight of 11 men who still could control their urine after radical prostatectomy regained their ability to achieve and maintain erections. Conversely, no man with urinary incontinence after radical prostatectomy had erectile function restored.
The researchers noted that the stem cell treatment was very well tolerated by all men, and described the procedure as safe.
While larger clinical trials are needed to confirm these results, autologous stem cells taken from a patient’s own fat tissue were able to restore erectile function in most of the men treated. This research suggests that men who do not lose urinary function may benefit from this procedure. On the other hand, men who become incontinent after radical prostatectomy may not benefit from this particular stem cell therapy. Randomized, placebo-controlled clinical trials will help clarify this issue. In the meantime, these results are encouraging news to thousands of men who suffer from permanent erectile dysfunction as a result of their radical prostatectomies.
Reference: Haahr, MK et al. (2016). Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine. 2016 Jan 19;5:204-10.

by admin | Sep 24, 2019 | Adipose, Stem Cell Research, Stem Cell Therapy
Crohn’s disease is a chronic illness that can affect any part of the gastrointestinal tract but mostly affects the small and large intestines. People with Crohn’s disease often have inflammation of the large bowel (Crohn’s disease is an inflammatory bowel disease or IBD). This colitis causes abdominal pain, cramping, diarrhea, along with bleeding and infections in the gastrointestinal tract. Crohn’s disease can interfere with a person’s ability to absorb nutrients, leading to malnutrition and weight loss. The medical community is debating whether it is possible to treat experimental colitis with fat-derived stem cells.
The standard medical treatment for Crohn’s disease involves one or more powerful drugs. When the disease flares up, patients usually must take steroids either orally or intravenously. They may also receive disease-modifying therapy such as immunomodulators and biologic medications. Many patients do enjoy remission once they receive these powerful drugs; however, side effects can be difficult to tolerate. Patients who cannot tolerate these powerful drugs or do not achieve disease remission may have to take steroids every day. Chronic steroid use has many severe and sometimes permanent side effects. If these treatments fail, patients may need to have surgery to remove a portion of their intestines that have been damaged by Crohn’s disease.
In an effort to find safe and effective treatments for Crohn’s disease, researchers have been testing stem cells in laboratory animals. In one study, scientists used a chemical to cause colon inflammation (colitis) in mice. This chemical causes many of the symptoms of humans with Crohn’s disease experience such as diarrhea, tissue damage, and weight loss. The researchers then treated some of the mice with mesenchymal stem cells gathered from human fat tissue (adipose) to see if stem cells could improve the symptoms.
Remarkably, human stem cell treatment reduced diarrhea, inflammation, and disease severity in mice with colitis. The chemical colitis caused mice to lose approximately 15 to 20% of their body weight. Mice that received stem cell treatment regained most of the weight they had lost. Researchers also noted that mice treated with adipose-derived mesenchymal stem cells lived significantly longer than those that did not receive stem cell treatment.
Of course, this research was performed in laboratory animals, but it lays important groundwork for testing in humans. Indeed, since the publication of this report, researchers have been able to show that adipose-derived stem cells helped patients with Crohn’s disease. This exciting work will no doubt lead to future studies that may help pave the way to wider use of stem cells in the treatment of inflammatory bowel disease, such as Crohn’s disease.
Reference: Gonzalez, M. (2009). Adipose-Derived Mesenchymal Stem Cells Alleviate Experimental Colitis by Inhibiting Inflammatory and Autoimmune Responses. Gastroenterology. Volume 136, Issue 3, March 2009, Pages 978-989

by admin | Aug 21, 2019 | Adipose, Aesthetics, Hair Regrowth
Alopecia, better known as hair loss, is a cosmetic problem. People do not need hair on their scalp to survive. Nonetheless, people with thinning hair or hair loss often endure considerable distress and suffering. Hair loss can cause low self-esteem, symptoms of depression, and a diminished quality of life. So while hair loss may be a simple cosmetic, strictly speaking, many people with alopecia struggle with an ongoing and serious problem.
Unfortunately, there are few effective treatments for hair loss. The two main medical treatments for hair loss are minoxidil and finasteride. Finasteride is generally only useful for male pattern baldness. Both men and women can use minoxidil, but it, too, is only partially effective. Various surgeries can be used to treat hair loss such as hair transplantation, scalp reduction, and scalp expansion, but patient satisfaction rates for these procedures are fairly low.
Stem cells that have been derived from fat tissue (i.e. adipose) secrete a number of beneficial chemicals called cytokines. These cytokines are important for wound healing and new blood vessel growth (i.e. angiogenesis). Cytokines released by adipose-derived stem cells are also able to stimulate hair follicles and induce the growth of hair. Based on these successes in the laboratory, dermatologists in Japan have used the substances secreted by adipose-derived stem cells to help people with hair loss.
Drs. Fukuoka, Narita, and Suga published a report detailing their successes in treating hair loss with proteins extracted from adipose-derived stem cells. A single hair loss treatment involves making a number of very small injections into the scalp. Each patient usually needs 6 to 8 treatment sessions, given once per month.
The doctors have performed this stem cell-based hair loss treatment on more than 1,000 patients and they have not encountered a single allergic reaction or infection. Indeed, no serious complications have occurred in their patients.
Not only is this stem cell-based hair loss treatment safe, but it is also apparently effective, as well. Patients have new growth of thin hair after two or three treatments, but this is minor and can usually only be detected by the doctors. After the fourth or fifth treatment, however, patients often notice new hair growth. By the sixth treatment, most patients can easily see new hair growth.
To confirm the effectiveness of their treatment, the doctors performed a half-side comparison test. In this test, they injected the stem cell-based hair loss treatment on one side of the scalp and injected saline on the other. The side of the scalp that received the stem cell extract had significantly more hair growth than the saline-treated side. This is strong evidence that the treatment is effective.
Reference: Fukuoka H. et al. (2017). Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. 2017;12(7):531-534.

by admin | Feb 4, 2019 | Adipose, Mesenchymal Stem Cells, Stem Cell Research, Stem Cell Therapy
Spinal cord injury is severe neurological condition in which
the major mode of transmission between the brain and the body is disrupted.
When higher levels of the spinal cord are injured, for example, in the neck,
the injury can be immediately fatal. Those who survived spinal cord injury are
often left paralyzed and at risk for a number of comorbid conditions
such as pneumonia, depression, skin ulceration infection, urinary tract
infections, and pain.
If patients who sustain spinal cord injury can receive
medical treatment quickly, physicians may administer glucocorticoids to help
reduce swelling around the injury and preserve spinal cord function. Patients
may also undergo therapeutic
hypothermia (a.k.a. targeted temperature management, whole body cooling),
also to help reduce inflammation and prevent scar tissue from forming around
the damaged spinal cord.
After the first few days to weeks after spinal cord injury,
not much can be done to change the outcome of the disease. Patients may undergo
intensive physical, occupational, and speech therapy to help regain function,
but more often than not the neurological deficits are mostly permanent. Hence,
researchers are feverishly searching for ways to treat spinal cord injury and,
by extension, prevent or reduce paralysis and other chronic complications.
Mesenchymal stem cells are an intriguing potential therapy
for spinal cord injury. These cells can easily be obtained from many different
tissues including bone marrow and fat among others. In animals, mesenchymal stem
cells have been shown to improve changes that occur during spinal cord injury,
namely the regeneration
and strengthening of nerve cells in the spinal cord. Research
has also shown how adipose-derived stem cells are a potential option for those
with neurological conditions such as spinal cord injury.
To test this possible effect in humans, researchers collected
mesenchymal stromal (stem) cells from patients with spinal cord injury in
their upper back (i.e. thoracic spinal cord). Researchers then prepared and administered
those cells back into the cerebrospinal fluid of the same patients. Each
patient received two or three injections of approximately 1,000,000 cells per
kilogram body weight. There were no adverse effects of the treatment for up to
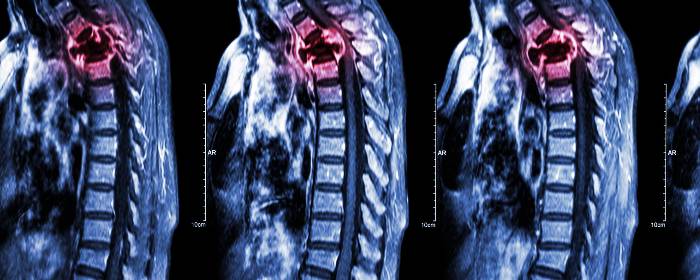
two years after injection. MRI imaging showed no abnormalities resulting from
stem cell infusion. While the authors write that there were too few patients to
make any firm conclusions about the efficacy of the treatment, they were
strongly encouraged by the safety of the procedure. In fact, they use these
results to begin a placebo-controlled clinical trial.
Reference
Satti et al. (2016). Autologous mesenchymal stromal cell
transplantation for spinal cord injury: A Phase I pilot study. International Society for Cellular Therapy,
18(4),518-522.




 St. Petersburg, Florida
St. Petersburg, Florida
