
by admin | Feb 20, 2017 | Studies
Diabetic patients from deficiencies in insulin, a hormone that is critical for regulating blood sugar levels. Researchers have recently shown how combining mesenchymal stem cells with bone marrow cells can induce the regeneration of cells that secrete insulin and thereby restore normal levels of blood sugar and blood insulin.
The insulin deficiency that occurs in diabetes differs based on the type of diabetes that a patient has. In type 1 diabetes, cells in the pancreas that produce insulin, known as beta-cells, are destroyed, preventing the normal production and secretion of insulin. In type 2 diabetes, however, insulin is still produced and secreted, but insulin sensitivity is reduced, meaning that the body does not respond properly to the presence of insulin in the blood. In both cases, there is a reduction in the number of properly functioning beta-cells.
Previous research has shown that adult bone marrow contains cells that can enhance the regeneration of beta-cells in diabetes. However, the findings have been countered by other studies that have not found this effect. In the current study, researchers aimed to determine whether bone marrow cells that are injected in combination with mesenchymal stem cells could increase the amount of functional beta-cells in diabetes. The scientists were also interested in whether any increase in functional Beta cells that may be observed as a result of this intervention would have the practical effect of restoring normal blood insulin and blood glucose levels in diabetes.
Though injecting just bone marrow cells or just mesenchymal stem cells did not impact beta-cell number or blood insulin or blood glucose levels, the combination of the two did. After a single injection, researchers observed tissue regeneration. Importantly, the new beta-cells were generated by the recipient, as no donor beta-cells were identified in recipients. Thus, the bone marrow cell and stem cell combination did not simply replace cells but instead instigated the regeneration of cells. Further, because blood insulin and blood glucose levels were restored after these injections, the newly generated beta-cells were not only present but also functional.
Another positive outcome of this procedure was that there was no immune response initiated against the new beta-cells, suggesting that these cells could survive in the long-term. These findings show the tremendous promise that stem cells have, especially when strategically combined with other interventions, to diabetes therapy.
Find out how stem cells provide regenerative therapy for Diabetes here.
Reference
Urban, V.S. et al. 2008. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells, 26, 244-253

by admin | Feb 10, 2017 | Studies
Researchers have now shown that stem cells that are genetically modified may be able to help in Parkinson’s disease by replacing the cells that are damaged in the disease. Their research was recently published in the journal CNS and Neurological Disorders – Drug Targets. Parkinson’s disease affects the stratum, a specific part of the brain and disrupts dopamine signaling. Based on what is known about the pathology of the disease, research into treatments has focused on how to restore proper dopamine functioning. Most of these approaches have been pharmaceutical in nature, and though some of the treatments that have been developed have been helpful in the short-term, they unfortunately have not been effective in the long-term.
Stem cells have shown promise for treating a number of clinical conditions, in large part because they provide a means for replacing cells that may be damaged due to injury or disease. Based on a number of theoretical arguments that neural stem cells, and particularly genetically modified stem cells, could potentially help with Parkinson’s disease, researchers set out to determine the practicality of trying to implant these cells into the brain. What is particularly challenging about the endeavor is not physically putting the stem cells into the brain but getting the cells to survive, differentiate into brain cells, and integrate themselves into the brain in a way that allows them to function properly, replacing the function of those cells that have been lost due to disease.
The researchers found that the stem cells they used were able to integrate specifically into the stratum, the part of the brain that is preferentially affected by Parkinson’s disease. Further, the neural cells specifically differentiated into the types of cells that are lost in Parkinson’s disease. These findings show the promise for using specific types of stem cells to help with Parkinson’s disease. Unlike other approaches to the disease, which have had only short-term success, stem cell techniques provide the novel approach of completely replacing the cells that are lost and may therefore help restore the functions that are lost as a result of cell loss. Further research will need to explore this question of if genetically modified stem cells actually reverse the symptoms associated with Parkinson’s disease. If these cells are able to provide a way to restore function in the disease, they could contribute to important interventions for neurodegeneration.
See how stem cell treatment for Parkinson’s disease have shown beneficial in the management of symptoms.
Reference
Ziavra, D. et al. (2012). Neural stem cells transplanted in a mouse model of Parkinson’s disease differentiate to neuronal phenotypes and reduce rotational deficit. CNS & Neurological Disorders – Drug Targets, 11(7), 829-835.

by admin | Feb 6, 2017 | Studies
In a thoughtful review, Meng Liu and Zhong Chao Han discuss the reasons that mesenchymal stem cells derived from bone marrow, fat, and fetal tissue are attractive candidates for improving type 1 diabetes therapy. Though type 1 diabetes is often treated with insulin replacement therapy, transplanting islet cells from the pancreas has become a means for curing the disease. Nonetheless, islet transplantation has a number of limitations, most of which occur as a result of isolating the islet cells from the pancreas. While isolated, the cells are vulnerable to damage and death, and as a result, effective therapy often requires a series of transplants.
Because mesenchymal stem cells possess a number of important characteristics that are lost or absent in islet cells, scientists have posited that they may have potential in the treatment of diabetes. For instance, immune system reactions to transplants often make transplants unsuccessful. However, mesenchymal stem cells modulate the immune system such that they can minimize these immune reactions. Mesenchymal stem cells are also able to differentiate into a variety of cell lineages and to promote the development of new blood vessels. These features make mesenchymal stem cells appealing from the general perspective of transplantation, where the generation of new cells and new cell networks within tissue is critical.
Given that mesenchymal stem cells appear to be a promising candidate for diabetes therapy, researchers have pondered how best to leverage the potential of these stem cells for the specific purpose of reinstating insulin function in type 1 diabetics. According to this review, one leading notion is to generate insulin-producing cells from mesenchymal stem cells.
Though there appear to be a number of specific ways to achieve insulin-producing cells from mesenchymal stem cells, the researchers point to the technical challenges associated with these methods but remain optimistic about the potential to do so. By laying the conceptual groundwork for using mesenchymal stem cells for type 1 diabetes treatment, Liu and Chao pave the way for future researchers to develop specific research protocols for improving our understanding of how mesenchymal stem cells can be used in the development of these important therapies.
Learn about how stem cells can help treat severe Diabetes here.
Reference
Liu, M & Han, Z.C. (2008). Mesenchymal stem cells: biology and clinical potential in type 1 diabetes therapy. Journal of Cellular & Molecular Medicine, 12(4), 1155-1168.

by admin | Jan 31, 2017 | Studies
In recent years, stem cells have continued to show promise for helping combat a host of diseases, many of which relate to the brain. A recent study by Yoo-Hun Suh and colleagues has demonstrated that a specific type of stem cell could help with both the prevention and treatment of the neurodegenerative disease, Alzheimer’s.
Patients with Alzheimer’s disease lose a significant number of brain cells as a result of the disease, and the resulting damage to brain tissue is associated with cognitive and behavioral symptoms. The disease is best known for causing significant memory difficulties in its sufferers. Because stem cells offer a way to introduce new cells into the organ, they are obvious candidates for Alzheimer’s therapy.
In the current study, published in PLOS One, the researchers set out to determine if they could overcome the technical difficulty of implanting human adipose-derived stem cells into the brain and, if so, whether these cells could improve the symptoms and the physical hallmarks of Alzheimer’s disease.
The researchers achieved a number of notable results. First, they showed that the stem cells were able to penetrate the blood-brain barrier and migrate into the brain. Second, they demonstrated that a number of symptoms associated with Alzheimer’s disease improved with the administration of stem cells in a model of Alzheimer’s disease. Specifically, learning and memory deficits were reversed. Finally, the scientists found that the administration of stem cells was associated with reductions in the physiological markers of Alzheimer’s disease – namely, the amyloid plaques in the brain that are a signature of the disease, as well as the protein that contributes to these plaques, called A. The researchers conclude that the stem cells may help with the therapy of Alzheimer’s and could potentially help with prevention as well.
That one study could achieve a technical proof-of-concept of administering the stem cells to the brain while also simultaneously demonstrating an improvement in symptoms and physiology associated with Alzheimer’s disease is incredible in terms of the potential for stem cells in aid in the therapeutic interventions of this disease.
Learn more about stem cell therapy for Alzheimer’s disease here.
Reference
Kim, S. et al. (2012). The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLOS One, 7(9), e45757.

by admin | Jan 26, 2017 | Studies
Severe diabetics are often treated through a technique called islet transplantation, but this transplantation method has certain limitations. Researchers have recently demonstrated that that using a certain type of stem cell, called the mesenchymal stem cell, can improve the ability of islet transplants to improve diabetes in patients.
Those who suffer from Type 1 diabetes experience the destruction of beta cells in the pancreas. Islet transplantation is a means for reconstructing these cells that are lost during the progression of diabetes, but the long-term effects of traditional islet transplantation are limited, and the transplants often have to be performed a number of times on any given patient. Part of the problem is that the beta cells in the islets are easily damaged or killed once they are isolated for transplantation purposes and do not easily proliferate into other cells.
Unlike islets, mesenchymal stem cells are less vulnerable, highly proliferative, and differentiate into several cell types. They also have the added benefits of promoting healthy vasculature and immune activity. Given these benefits of mesenchymal stem cells, scientists hypothesized that combining the function of beta cells with the abilities of mesenchymal stem cells would produce a more effective therapy for diabetes than islet transplantation alone. To achieve this result, Shoichiro Sumi’s team decided to fuse these cell types together.
In a study published in PLOS One, researchers demonstrated how to successfully overcome the technical difficulty of fusing mesenchymal stem cells to islet cells for the purposes of treating diabetes. The fused cells were associated with markers that indicated that the cells were less susceptible to death than islet cells are alone and that they also proliferated more than islet cells generally do.
Having cleared the technical hurdle of fusing islet cells with mesenchymal stem cells and showing that this technique is successful in creating cells that are more robust than islet cells, researchers are now poised to test the ability of these cells to improve therapeutic interventions for those with severe diabetes. If the researchers can show that fewer transplants or longer-term results can be achieved with these fused cells, islet transplantation for diabetics may be transformed.
Learn more about how stem cells provide regenerative therapy for Diabetes here.
Reference
Yanai, G. et al (2013). Electrofusion of mesenchymal stem cells and islet cells for diabetes therapy: a rat model. PLOS One, 8(5), 1-9.

by admin | Jan 16, 2017 | Studies
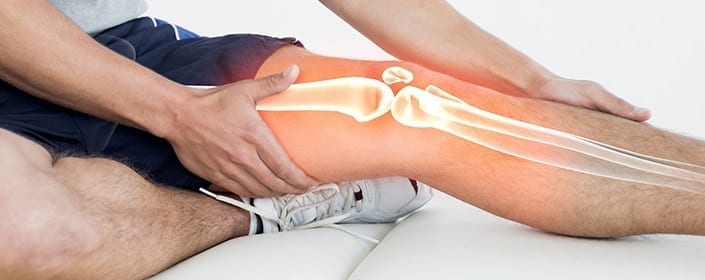
In recent years, there have been a number of advances in stem cell research and in the various ways that these cells can be best employed to improve the health of patients. One of the medical areas that shows promise for stem cell therapies is orthopedics, with stem cell therapies being developed to help with tissue, cartilage, and bone repair. A recent review by Anish Majumdar and colleagues conveyed the progress that has been made specifically in the use of stem cells for the repair of cartilage in osteoarthritis.
Patients with osteoarthritis experience degeneration of their connective tissues, which progresses as they get older. While osteoarthritis is often diagnosed in older patients, athletes also often endure osteoarthritis after injuring themselves while playing sports. In osteoarthritis, tissue known as articular cartilage is particularly susceptible to injury and unfortunately does not heal as well as other tissues because it does not have the vasculature required to supply the tissue with the nutrients required for significant growth and recovery.
When articular cartilage is damaged, surgery is often employed in an attempt to correct the damage, and pharmaceuticals are sometimes prescribed for discomfort. However, these interventions do not tend to achieve satisfying results. Because bone marrow stromal cells, or bone marrow-derived mesenchymal stem cells, naturally differentiate into the cells that make up cartilage, it has been suggested that these stem cells could improve outcomes for those with osteoarthritis.
These particular stem cells have other advantages, including that their isolation is relatively simple and that they easily proliferate, adds to their attractiveness as a candidate for cartilage repair. Their ability to suppress the immune system and prevent inflammation makes them more likely than many other cell types to be safe when added to the cartilage. As such, according to this review, a number of researchers have reported that their injections of these stem cells in patients with osteoarthritis have not caused any problems related to safety.
These findings include stem cells leading to improvements in clinical symptoms and quality of life in those with osteoarthritis, as well as the filling of the defect area and reduction in pain.
Researchers have also reported that these stem cells are effective from a therapeutic standpoint when administered to osteoarthritis patients. A number of specific findings on the success of bone marrow-derived mesenchymal stem cells in cartilage repair demonstrate that stem cells could revolutionize therapeutic strategies for this type of tissue damage. These findings include stem cells leading to improvements in clinical symptoms and quality of life in those with osteoarthritis, as well as the filling of the defect area and reduction in pain.
See why more and more athletes are turning to stem cell therapy here.
Reference
Gupta, P.K., Das, A.K., Chullikana, A., & Majumdar, A.S. (2012). Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Research & Therapy, 3(25), 1-9.






 St. Petersburg, Florida
St. Petersburg, Florida
