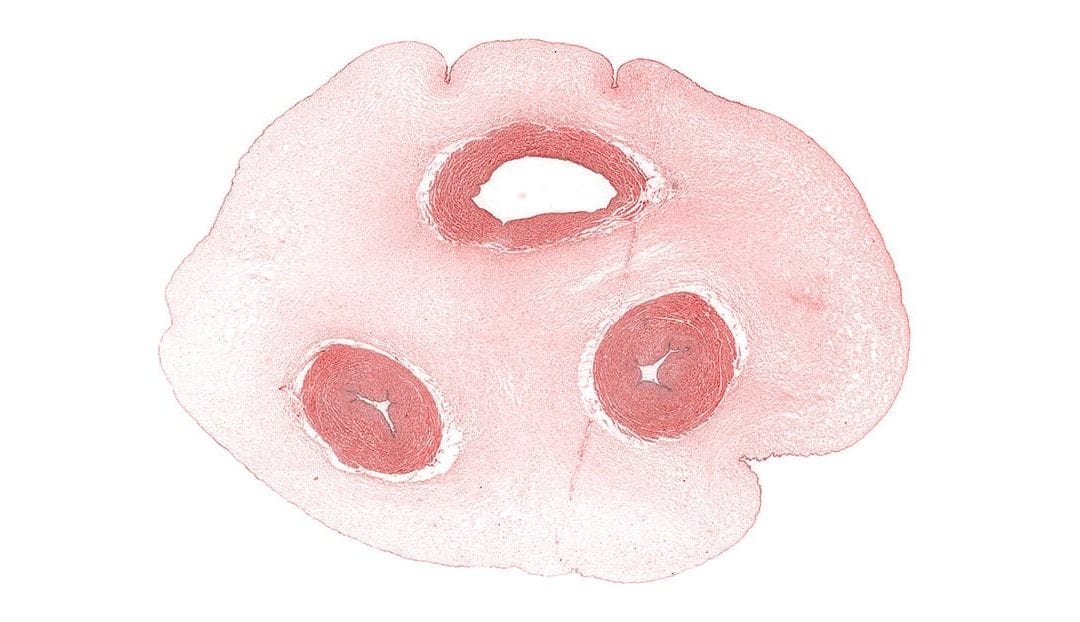
Wharton’s jelly is a rich source of stem cells, but some researchers believe its value is sometimes unappreciated. A recent review published in Stem Cells Translational Medicine synthesized an abundance of information on what is known about Wharton’s jelly. Because scientists often use inconsistent language to discuss observations and research results pertaining to Wharton’s jelly, the authors also proposed a nomenclature to help improve transparency related to methods and findings that involve Wharton’s jelly.
According to the authors, there are a number of advantages of stem cells that come from Wharton’s jelly that are making it a popular source of these cells. One critical benefit is the noninvasive nature of the stem cell collection. Because Wharton’s jelly is collected from tissue that would otherwise be discarded after the delivery of a baby, collecting the stem cells does not require its own invasive procedure.
Another apparent benefit of Wharton’s jelly is its therapeutic efficacy. Because basic science studies have demonstrated the potential for Wharton’s jelly-derived mesenchymal stromal cells to outperform other stem cell types in their ability to treat disease, these cells are now being tested in a number of clinical trials.
For progress to be made with Wharton’s jelly-derived stem cells, it is important that researchers have a common language with which to talk about the human umbilical cord, which is the source of these cells. While this connective tissue is simpler than other types of connective tissue, it is still unclear how to differentiate the different types of cells that come from Wharton’s jelly. More precise language and definitions should help overcome this problem.

 St. Petersburg, Florida
St. Petersburg, Florida
