Spinal Cord Stimulation
Now covered by Medicare and Insurance
What is Spinal Cord Stimulation?
How do Spinal Cord Stimulators work?
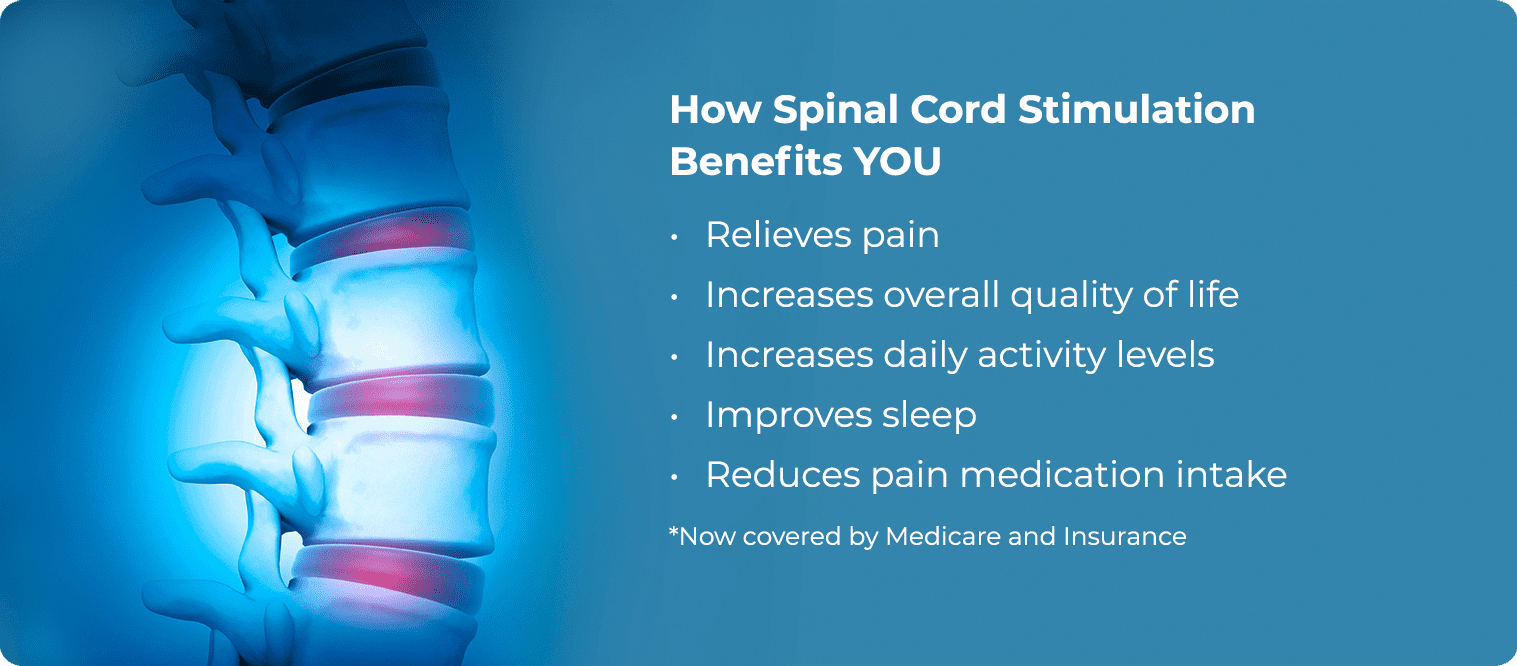

What Conditions do Spinal Cord Stimulation Treat?
Spinal Cord Stimulators are used to treat or manage many different types of chronic pain including:
- Back pain (especially after a failed back surgery)
- Injuries to the spinal cord
- Complex Regional Pain Syndrome (CRPS)
- Post-surgical pain
- Nerve related pain
- Arachnoiditis (painful inflammation of the thin layer (arachnoid) that covers the brain and spinal cord
- Pain after amputation
- Visceral abdominal pain and perineal pain
- Peripheral Vascular Disease
Who is a candidate for Spinal Cord Stimulation? Those who:
- Have had non-surgical options fail to provide adequate pain relief
- Do not have psychiatric problems that would interfere with the effectiveness of the treatment
- Do not have bleeding problems, infections, or pregnant
- Have had a positive response from the Spinal Cord Stimulator Trial
Spinal Cord Stimulator Trial
At Stemedix, the Spinal Cord Stimulator Trial and implantation procedures are performed by a Board-Certified Anesthesiologist who has further specialized in Interventional Pain Management.
Before the Procedure
What is needed:
- A complete a Medical History Form
- Copies of all imaging studies related to your source of pain
- Submit a current list of all medications/supplements that you are taking
- Halt taking any blood-thinning medications/supplements before the procedure
The Trial Procedure
The procedures are performed by the physician using a fluoroscopy machine. The fluoroscope allows the physician to see the precise location to place the electrodes in the epidural space, which is located between the spinal cord and the vertebrae.
The trial procedure only requires a small incision in your lower back to place the electrodes.The generator will be placed on the outside usually worn by you around your waist. You will be required to test the device for about a week to see if it relieves your pain. If your pain is reduced by at least 50%, the trial is considered to be successful and you will be scheduled to have the device implanted.
If the trial is unsuccessful, the electrodes can easily be removed in our office without causing any damage to the spinal cord or nerves. The physician will move to potential other interventions available to help control your pain.
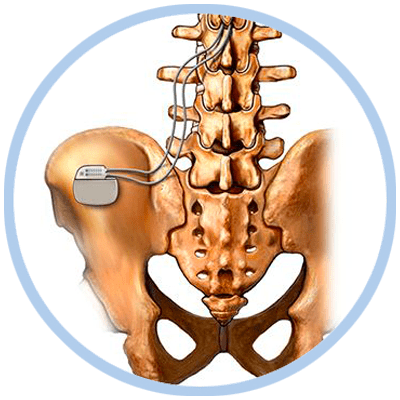
Spinal Cord Stimulator Implantation Procedure
The implantation procedure is performed much like the trial procedure except for the following:
- The generator is placed just under the skin in the area of the buttocks through a small incision.
- The trial electrodes are replaced and anchored with sutures to minimize movement.
After the Procedure
| Experience | Timeframe |
| Pain at the incision site | Several days after the procedure |
| Removal of dressings over the incision site | 3 days after the procedure |
| Incision healing | 2-4 weeks after the procedure |
| Light activity level | First 2 weeks after the procedure |
| Returning to work | 1-2 weeks after the procedure |
| Driving (with the stimulator turned off) | 1-2 weeks after the procedure |
Risks/Complications
Spinal Cord Stimulator Trial and Implantation Procedures are considered safe with a minimal risk for complications. Complications that have been seen are:
- Infection that may occur within the first 2-8 weeks after implantation
- Bleeding
- Device Migration of the electrodes moving from their original position. This may result in being less effective in relieving pain. If this occurs, a follow-up procedure would be indicated to readjust the electrodes.
- Device Failure from mechanical injury to the device from a fall, etc.
- Dural Puncture to the outer protective layer of the spinal cord/brain is inadvertently punctured and causes cerebrospinal fluid to leak out which may cause severe headaches.
- Spinal cord trauma is extremely rare and is a result causing nerve injury and paralysis.
Living with a Spinal Cord Stimulator

- CT Scans and X-rays – As long as the unit is powered “OFF,” these procedures are safe. It is always a good idea to inform the technician that you have got an implanted spinal cord stimulator.
- MRIs – No. MRIs are not always compatible with Spinal Cord Stimulator Devices. Check with the physician or technician to see if the device model that you have is compatible first.
- Airport Security – Your stimulator device will be detected while going through airport security. You may have to power “OFF” the device before going through. You will be provided with an identification card to present to airport security for this purpose.
- Swimming – It is okay to swim with the implanted device. However, you can NOT get the temporary device wet. You will need to avoid showers or baths during the trial period. Sponge-bathing is okay.

 St. Petersburg, Florida
St. Petersburg, Florida
